Mohave Ground Squirrel Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mammals of the California Desert
MAMMALS OF THE CALIFORNIA DESERT William F. Laudenslayer, Jr. Karen Boyer Buckingham Theodore A. Rado INTRODUCTION I ,+! The desert lands of southern California (Figure 1) support a rich variety of wildlife, of which mammals comprise an important element. Of the 19 living orders of mammals known in the world i- *- loday, nine are represented in the California desert15. Ninety-seven mammal species are known to t ':i he in this area. The southwestern United States has a larger number of mammal subspecies than my other continental area of comparable size (Hall 1981). This high degree of subspeciation, which f I;, ; leads to the development of new species, seems to be due to the great variation in topography, , , elevation, temperature, soils, and isolation caused by natural barriers. The order Rodentia may be k., 2:' , considered the most successful of the mammalian taxa in the desert; it is represented by 48 species Lc - occupying a wide variety of habitats. Bats comprise the second largest contingent of species. Of the 97 mammal species, 48 are found throughout the desert; the remaining 49 occur peripherally, with many restricted to the bordering mountain ranges or the Colorado River Valley. Four of the 97 I ?$ are non-native, having been introduced into the California desert. These are the Virginia opossum, ' >% Rocky Mountain mule deer, horse, and burro. Table 1 lists the desert mammals and their range 1 ;>?-axurrence as well as their current status of endangerment as determined by the U.S. fish and $' Wildlife Service (USWS 1989, 1990) and the California Department of Fish and Game (Calif. -
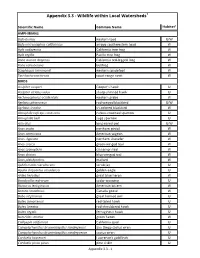
Appendix 3.3 - Wildlife Within Local Watersheds1
Appendix 3.3 - Wildlife within Local Watersheds1 2 Scientific Name Common Name Habitat AMPHIBIANS Bufo boreas western toad U/W Bufo microscaphus californicus arroyo southwestern toad W Hyla cadaverina California tree frog W Hyla regilla Pacific tree frog W Rana aurora draytonii California red-legged frog W Rana catesbeiana bullfrog W Scaphiopus hammondi western spadefoot W Taricha torosa torosa coast range newt W BIRDS Accipiter cooperi Cooper's hawk U Accipiter striatus velox sharp-shinned hawk U Aechmorphorus occidentalis western grebe W Agelaius phoeniceus red-winged blackbird U/W Agelaius tricolor tri-colored blackbird W Aimophila ruficeps canescens rufous-crowned sparrow U Aimophilia belli sage sparrow U Aiso otus long-eared owl U/W Anas acuta northern pintail W Anas americana American wigeon W Anas clypeata northern shoveler W Anas crecca green-winged teal W Anas cyanoptera cinnamon teal W Anas discors blue-winged teal W Anas platrhynchos mallard W Aphelocoma coerulescens scrub jay U Aquila chrysaetos canadensis golden eagle U Ardea herodius great blue heron W Bombycilla cedrorum cedar waxwing U Botaurus lentiginosus American bittern W Branta canadensis Canada goose W Bubo virginianus great horned owl U Buteo jamaicensis red-tailed hawk U Buteo lineatus red-shouldered hawk U Buteo regalis ferruginous hawk U Butorides striatus green heron W Callipepla californica California quail U Campylorhynchus brunneicapillus sandiegensis San Diego cactus wren U Campylorhynchus brunneicapillus sandiegoense cactus wren U Carduelis lawrencei Lawrence's -

Mammal Species Native to the USA and Canada for Which the MIL Has an Image (296) 31 July 2021
Mammal species native to the USA and Canada for which the MIL has an image (296) 31 July 2021 ARTIODACTYLA (includes CETACEA) (38) ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BALAENIDAE - bowheads and right whales 1. Balaena mysticetus – Bowhead Whale BALAENOPTERIDAE -rorqual whales 1. Balaenoptera acutorostrata – Common Minke Whale 2. Balaenoptera borealis - Sei Whale 3. Balaenoptera brydei - Bryde’s Whale 4. Balaenoptera musculus - Blue Whale 5. Balaenoptera physalus - Fin Whale 6. Eschrichtius robustus - Gray Whale 7. Megaptera novaeangliae - Humpback Whale BOVIDAE - cattle, sheep, goats, and antelopes 1. Bos bison - American Bison 2. Oreamnos americanus - Mountain Goat 3. Ovibos moschatus - Muskox 4. Ovis canadensis - Bighorn Sheep 5. Ovis dalli - Thinhorn Sheep CERVIDAE - deer 1. Alces alces - Moose 2. Cervus canadensis - Wapiti (Elk) 3. Odocoileus hemionus - Mule Deer 4. Odocoileus virginianus - White-tailed Deer 5. Rangifer tarandus -Caribou DELPHINIDAE - ocean dolphins 1. Delphinus delphis - Common Dolphin 2. Globicephala macrorhynchus - Short-finned Pilot Whale 3. Grampus griseus - Risso's Dolphin 4. Lagenorhynchus albirostris - White-beaked Dolphin 5. Lissodelphis borealis - Northern Right-whale Dolphin 6. Orcinus orca - Killer Whale 7. Peponocephala electra - Melon-headed Whale 8. Pseudorca crassidens - False Killer Whale 9. Sagmatias obliquidens - Pacific White-sided Dolphin 10. Stenella coeruleoalba - Striped Dolphin 11. Stenella frontalis – Atlantic Spotted Dolphin 12. Steno bredanensis - Rough-toothed Dolphin 13. Tursiops truncatus - Common Bottlenose Dolphin MONODONTIDAE - narwhals, belugas 1. Delphinapterus leucas - Beluga 2. Monodon monoceros - Narwhal PHOCOENIDAE - porpoises 1. Phocoena phocoena - Harbor Porpoise 2. Phocoenoides dalli - Dall’s Porpoise PHYSETERIDAE - sperm whales Physeter macrocephalus – Sperm Whale TAYASSUIDAE - peccaries Dicotyles tajacu - Collared Peccary CARNIVORA (48) CANIDAE - dogs 1. Canis latrans - Coyote 2. -

GOOSEBERRYLEAF GLOBEMALLOW Sphaeralcea Grossulariifolia (Hook
GOOSEBERRYLEAF GLOBEMALLOW Sphaeralcea grossulariifolia (Hook. & Arn.) Rydb. Malvaceae – Mallow family Corey L. Gucker & Nancy L. Shaw | 2018 ORGANIZATION NOMENCLATURE Sphaeralcea grossulariifolia (Hook. & Arn.) Names, subtaxa, chromosome number(s), hybridization. Rydb., hereafter referred to as gooseberryleaf globemallow, belongs to the Malveae tribe of the Malvaceae or mallow family (Kearney 1935; La Duke 2016). Range, habitat, plant associations, elevation, soils. NRCS Plant Code. SPGR2 (USDA NRCS 2017). Subtaxa. The Flora of North America (La Duke 2016) does not recognize any varieties or Life form, morphology, distinguishing characteristics, reproduction. subspecies. Synonyms. Malvastrum coccineum (Nuttall) A. Gray var. grossulariifolium (Hooker & Arnott) Growth rate, successional status, disturbance ecology, importance to animals/people. Torrey, M. grossulariifolium (Hooker & Arnott) A. Gray, Sida grossulariifolia Hooker & Arnott, Sphaeralcea grossulariifolia subsp. pedata Current or potential uses in restoration. (Torrey ex A. Gray) Kearney, S. grossulariifolia var. pedata (Torrey ex A. Gray) Kearney, S. pedata Torrey ex A. Gray (La Duke 2016). Seed sourcing, wildland seed collection, seed cleaning, storage, Common Names. Gooseberryleaf globemallow, testing and marketing standards. current-leaf globemallow (La Duke 2016). Chromosome Number. Chromosome number is stable, 2n = 20, and plants are diploid (La Duke Recommendations/guidelines for producing seed. 2016). Hybridization. Hybridization occurs within the Sphaeralcea genus. -

L O U I S I a N A
L O U I S I A N A SPARROWS L O U I S I A N A SPARROWS Written by Bill Fontenot and Richard DeMay Photography by Greg Lavaty and Richard DeMay Designed and Illustrated by Diane K. Baker What is a Sparrow? Generally, sparrows are characterized as New World sparrows belong to the bird small, gray or brown-streaked, conical-billed family Emberizidae. Here in North America, birds that live on or near the ground. The sparrows are divided into 13 genera, which also cryptic blend of gray, white, black, and brown includes the towhees (genus Pipilo), longspurs hues which comprise a typical sparrow’s color (genus Calcarius), juncos (genus Junco), and pattern is the result of tens of thousands of Lark Bunting (genus Calamospiza) – all of sparrow generations living in grassland and which are technically sparrows. Emberizidae is brushland habitats. The triangular or cone- a large family, containing well over 300 species shaped bills inherent to most all sparrow species are perfectly adapted for a life of granivory – of crushing and husking seeds. “Of Louisiana’s 33 recorded sparrows, Sparrows possess well-developed claws on their toes, the evolutionary result of so much time spent on the ground, scratching for seeds only seven species breed here...” through leaf litter and other duff. Additionally, worldwide, 50 of which occur in the United most species incorporate a substantial amount States on a regular basis, and 33 of which have of insect, spider, snail, and other invertebrate been recorded for Louisiana. food items into their diets, especially during Of Louisiana’s 33 recorded sparrows, Opposite page: Bachman Sparrow the spring and summer months. -
![Germination and Seedling Establishment of Spiny Hopsage (Grayia Spinosa [Hook.] Moq.)](https://docslib.b-cdn.net/cover/8079/germination-and-seedling-establishment-of-spiny-hopsage-grayia-spinosa-hook-moq-328079.webp)
Germination and Seedling Establishment of Spiny Hopsage (Grayia Spinosa [Hook.] Moq.)
AN ABSTRACT OF THE THESIS OF Nancy L. Shaw for the degree of Doctor of Philosophy in Crop and Soil Sciences presented on March 19, 1992 Title: Germination and Seedling Establishment of Spiny Hopsage (Grayia Spinosa [Hook.] Moq.) Abstract approved:_Redactedfor Privacy von r. ULdUe Reestablishment of spiny hopsage(Grayia spinosa [Hook.] Moq.) where depleted or lost on shrub steppe sites can improve forage, plant cover, and soil stabilization. The objectives of this study were to: 1) determine direct-seeding requirements; 2) develop optimum germination pretreatments; and 3) examine dormancy mechanisms in spiny hopsage fruits and seeds. The effects of seed source, planting date,and site preparation method onseed germination and seedling establishment (SE) were examined at Birds of Prey and Reynolds Creek in southwestern Idaho. Three seed sources were planted on rough or compact seedbeds on 4 dates in 1986-87 and 3 dates in 1987-88. Exposure to cool-moist environments improved spring SE from early fall (EF) and late fall (LF) plantings. Few seedlings emerged from early (ESp) or late spring (LSp) plantings. SE was low at: 1 site in 1986-87 and atboth sites in 1987-88, probably due to lack of precipitation. For the successful 1986-87 planting, seedling density was greater on rough compared to compact seedbeds in April andMay, possiblydue to improved microclimate conditions. Growth rate varied among seed sources, but seedlings developed a deep taproot (mean length 266 mm) with few lateral roots the first season. Seeds were planted on 3 dates in 1986-87 and 1987-88, andnylon bags containing seeds were planted on 4 dates each year to study microenvironment effects on germination (G), germination rate (GR), and SE. -

A Reciprocal Transplant Experiment with Winterfat (Krascheninnikovia Lanata) Melanie Barnes
University of New Mexico UNM Digital Repository Biology ETDs Electronic Theses and Dissertations 12-1-2009 The effect of plant source location on restoration success: a reciprocal transplant experiment with winterfat (Krascheninnikovia lanata) Melanie Barnes Follow this and additional works at: https://digitalrepository.unm.edu/biol_etds Recommended Citation Barnes, Melanie. "The effect of plant source location on restoration success: a reciprocal transplant experiment with winterfat (Krascheninnikovia lanata)." (2009). https://digitalrepository.unm.edu/biol_etds/4 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at UNM Digital Repository. It has been accepted for inclusion in Biology ETDs by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. The effect of plant source location on restoration success: a reciprocal transplant experiment with winterfat (Krascheninnikovia lanata) BY Melanie G. Barnes B.A., Biology, Reed College, 2001 DISSERTATION Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Biology The University of New Mexico Albuquerque, New Mexico December, 2009 DEDICATION In memory of my mother, Georgene Grace Barnes. The completion of this dissertation is also dedicated to my friends and family who have supported me in this endeavor and who taught me many things about life that gave me the perspective I needed to complete this work. I would like to thank Heather Simpson, Jerusha Reynolds, Terri Koontz, Nathan Abrahamson, Jeremy Barlow, Brittany Barker, Laura Calabrese, Jennifer Hollis, Maureen Peters, Helen Barnes, and Tom Barnes. Finally, I want to thank Lisa for her love and emotional support; it means the world to me. -

Phylogeny and Biogeography of the Pleistocene Holarctic Steppe and Semi-Desert Goosefoot Plant Krascheninnikovia Ceratoides
Flora 262 (2020) 151504 Contents lists available at ScienceDirect Flora journal homepage: www.elsevier.com/locate/flora Phylogeny and biogeography of the Pleistocene Holarctic steppe and semi- desert goosefoot plant Krascheninnikovia ceratoides T Anna Seidla,*, Ernesto Pérez-Collazosb, Karin Tremetsbergera, Mark Carinec, Pilar Catalánb, Karl-Georg Bernhardta a Institute of Botany, University of Natural Resources and Life Sciences, Gregor-Mendel-Straße 33, 1180 Vienna, Austria b Escuela Politécnica Superior de Huesca, Universidad de Zaragoza, Ctra. Cuarte Km 1, 22071 Huesca, Spain c Natural History Museum, Cromwell Rd, Kensington, London SW7 5BD, UK ARTICLE INFO ABSTRACT Edited by: Chennai Guest Editor Krascheninnikovia ceratoides (Chenopodiaceae) is a steppe and semi-desert plant with two subspecies, K. cer- Keywords: atoides subsp. ceratoides, which is widespread in Eurasia, and K. ceratoides subsp. lanata, which grows in western Altai Mountains and central North America. A few disjunct populations of K. ceratoides subsp. ceratoides are found in Anatolia, Irano-Turanian element Europe and North Africa to the west of its otherwise continuous Eurasian distribution. To understand the evo- rDNA and plastid data lutionary history of this characteristic steppe and semi-desert plant, we analysed its phylogeny and biogeo- phylogeography graphy. We sequenced several loci including ITS, ETS and the chloroplast intergenic spacer regions atpB-rbcL, molecular dating rpl32-trnL and trnL-trnF to establish a time-calibrated phylogeny and reconstruct intraspecific relationships. Pleistocene Furthermore, we identified the ploidy level of individuals. While diploid, tetraploid and hexaploid individuals have been reported in the literature, we were only able to find diploids and tetraploids. The diploids were found in the east of Mongolia, Kazakhstan, Kyrgyzstan, Russia and the USA. -

Why Birds Are So Named
27 WHY BIRDS ARE SO NAMED. BY KATIE M. ROADS. “What’s in a name ?” Would some of the names of our birds suit one as well as another, or, as in other branches of science, has there been some significance attached to them or so1n.echaracteristic described by them ? While some birds rest content with one name, some have such marked pecularities as to attract the attention of different per- sons and each person has given his own interpretatioa of these by giving a name of his own. This may account for the 124 different names for thbeFlicker as complcid by Prank L. Burns. in The Wilson Bulletin No. 31. While all birds have not this motley array of names, the majority are supplied with several. In the following incomplete list it will be observed that the names employed involve practically every part of the bird’s external anatomy. The color involves the main color of the bird,as well as the colo’r of the head, the back, the wings, the tail, the under parts, the sides, the biK,and even peculiarities in markings. The shape and length of tail and bill, peculiarities of feet and Iegs, the pIace it frequents, the call notes, the song, the imitation in either form, color, or notes, and other things, including persons and places. While many of these names are more or I’ess useful in describing the bird, some of them are distinctly misleading or misnomers. It will be impossible to collate all names which every bird may be or may have been called by, therefore it seems wise to limit this paper to the vernacular or English names in gene.r- al use and of recognized standing in ornithological literature. -

General Biological Resources Assessment
GENERAL BIOLOGICAL RESOURCES ASSESSMENT APN 0405-06-251 HESPERIA, SAN BERNARDINO COUNTY, CALIFORNIA (Township 4 North, Range 5 West, Section 14) Preparedfor: 55555 Amargosa Rod, LLC Prepared by: RCA Associates, Inc. 15555 Main Street, #D4-235 Hesperia, California 92345 (760) 596-0017 Principal Investigator: Randall Arnold, Senior Biologist RCA ):) <► <, ASSOCIATES.INC Project: #2019-18 January 30, 2020 TITLE PAGE Date Report Written: January 30, 2020 Date Field Work Completed: January 29, 2020 Report Title: General Biological Resources Assessment Assessor's Parcel Number: 0405-062-51 Prepared for: 55555 Amargosa Road, LLC Principal Investigators: Randall C. Arnold, Jr., Senior Biologist Contact Information: Randall C. Arnold, Jr. RCA Associates, Inc. 15555 Main Street, #D4-235 Hesperia, CA 92345 (760) 596-0017 [email protected] www .rcaassociatesllc.com Table of Contents Section Page 1.0 Introduction and Summary 2.0 Existing Conditions 2 3.0 Methodologies 4 4.0 Literature Search 6 5.0 Results 8 5.1 General Biological Resources 9 5.2 Federal and State Listed Species 9 5.3 Wildlife Species of Special Concern 9 5.4 Jurisdictional Waters and Riparian Habitat 12 5.5 Protected Plants 12 6.0 Impacts and Mitigation Measures 13 6.1 General Biological Resources 13 6.2 Federal and State Listed Species and Species of 13 Special Concern 7.0 Conclusions and Recommendations 14 8.0 Bibliography 15 Certification 17 Appendix A - Tables and Figures Appendix B - Regulatory Content 1.0 INTRODUCTION AND SUMMARY Biological surveys were conducted on a parcel which is 20.8-acres in size located north of Main Street and west of Amargosa Road in the City of Hesperia, California (Townsrup 4 North, Range 5 West, Section 14, USGS Hesperia, California Quadrangle, 1956) (Figures 1, 2, 3, and 4). -

Origin and Age of Australian Chenopodiaceae
ARTICLE IN PRESS Organisms, Diversity & Evolution 5 (2005) 59–80 www.elsevier.de/ode Origin and age of Australian Chenopodiaceae Gudrun Kadereita,Ã, DietrichGotzek b, Surrey Jacobsc, Helmut Freitagd aInstitut fu¨r Spezielle Botanik und Botanischer Garten, Johannes Gutenberg-Universita¨t Mainz, D-55099 Mainz, Germany bDepartment of Genetics, University of Georgia, Athens, GA 30602, USA cRoyal Botanic Gardens, Sydney, Australia dArbeitsgruppe Systematik und Morphologie der Pflanzen, Universita¨t Kassel, D-34109 Kassel, Germany Received 20 May 2004; accepted 31 July 2004 Abstract We studied the age, origins, and possible routes of colonization of the Australian Chenopodiaceae. Using a previously published rbcL phylogeny of the Amaranthaceae–Chenopodiaceae alliance (Kadereit et al. 2003) and new ITS phylogenies of the Camphorosmeae and Salicornieae, we conclude that Australia has been reached in at least nine independent colonization events: four in the Chenopodioideae, two in the Salicornieae, and one each in the Camphorosmeae, Suaedeae, and Salsoleae. Where feasible, we used molecular clock estimates to date the ages of the respective lineages. The two oldest lineages both belong to the Chenopodioideae (Scleroblitum and Chenopodium sect. Orthosporum/Dysphania) and date to 42.2–26.0 and 16.1–9.9 Mya, respectively. Most lineages (Australian Camphorosmeae, the Halosarcia lineage in the Salicornieae, Sarcocornia, Chenopodium subg. Chenopodium/Rhagodia, and Atriplex) arrived in Australia during the late Miocene to Pliocene when aridification and increasing salinity changed the landscape of many parts of the continent. The Australian Camphorosmeae and Salicornieae diversified rapidly after their arrival. The molecular-clock results clearly reject the hypothesis of an autochthonous stock of Chenopodiaceae dating back to Gondwanan times. -

Color Plant 2.P65
AG 510 USDA-ARS-Forage and Range Research Lab, Logan, Utah, in conjunction with Utah State University Extension PLANTING GUIDE 105 Writers and Compilers Kevin Jensen, Howard Horton, Ron Reed, Ralph Whitesides, and USDA-ARS-FRRL Utah State University, Logan Utah. 435-797-3066, E-mail: [email protected] Contributors Extension Specialist – Robert Newhall, Plants, Soils, Biometeorology Dept. Utah State University, 435-797-2183, E-mail: [email protected] Fertilization – Dr. Richard Koenig, Plants, Soils, and Biometeorology Dept. Utah State University, 435-797-2278, E-mail: [email protected] Irrigation – Dr. Robert Hill, Biology and Irrigation Engineering Dept. Utah State University, 435-797-1248, E-mail: [email protected] Weed Control – Dr. Steven Dewey, Plants, Soils, and Biometeorology Dept. Utah State University, 435-797-2256, E-mail: [email protected] Seed Quality – Dr. Stanford Young, Plants, Soils, and Biometeorology Dept. Utah State University, 435-797-2082, E-mail: [email protected] Riparian/Wetland Systems – Chris Hoag, Wettland Plant Ecologist, Interagency Riparian/Wetland Project, Plant Materials Center, USDA-NRCS, Aberdeen, Idaho Plant Materials Specialist and Riparian/Wetland Systems – Dan Ogle, USDA-NRCS, Boise, Idaho, 208-378-5730 Major References Consulted Cool-Season Forage Grass Monograph. 1996. Editors L.E. Moser, D.R. Buxton, and M.D. Casler. American Society of Agronomy Number 34. Alberta Forage Manual. 1992. Print Media Branch, Alberta Agriculture, 7000-113 Street, Edmonton, Alberta, T6H 5T6. Forages Volume 1: An Introduction to Grassland Agriculture. 1995. Editors R.F. Barnes, D.A. Miller, and C.J. Miller. Iowa State University Press. USDA, NRCS 1999.