Calculations There Are a Variety of Calculations That You Will Be Called Upon to Do Routinely in the Laboratory. Herein We Pres
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basic Statistics Range, Mean, Median, Mode, Variance, Standard Deviation
Kirkup Chapter 1 Lecture Notes SEF Workshop presentation = Science Fair Data Analysis_Sohl.pptx EDA = Exploratory Data Analysis Aim of an Experiment = Experiments should have a goal. Make note of interesting issues for later. Be careful not to get side-tracked. Designing an Experiment = Put effort where it makes sense, do preliminary fractional error analysis to determine where you need to improve. Units analysis and equation checking Units website Writing down values: scientific notation and SI prefixes http://en.wikipedia.org/wiki/Metric_prefix Significant Figures Histograms in Excel Bin size – reasonable round numbers 15-16 not 14.8-16.1 Bin size – reasonable quantity: Rule of Thumb = # bins = √ where n is the number of data points. Basic Statistics range, mean, median, mode, variance, standard deviation Population, Sample Cool trick for small sample sizes. Estimate this works for n<12 or so. √ Random Error vs. Systematic Error Metric prefixes m n [n 1] Prefix Symbol 1000 10 Decimal Short scale Long scale Since 8 24 yotta Y 1000 10 1000000000000000000000000septillion quadrillion 1991 7 21 zetta Z 1000 10 1000000000000000000000sextillion trilliard 1991 6 18 exa E 1000 10 1000000000000000000quintillion trillion 1975 5 15 peta P 1000 10 1000000000000000quadrillion billiard 1975 4 12 tera T 1000 10 1000000000000trillion billion 1960 3 9 giga G 1000 10 1000000000billion milliard 1960 2 6 mega M 1000 10 1000000 million 1960 1 3 kilo k 1000 10 1000 thousand 1795 2/3 2 hecto h 1000 10 100 hundred 1795 1/3 1 deca da 1000 10 10 ten 1795 0 0 1000 -

Time and Frequency Users' Manual
,>'.)*• r>rJfl HKra mitt* >\ « i If I * I IT I . Ip I * .aference nbs Publi- cations / % ^m \ NBS TECHNICAL NOTE 695 U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Time and Frequency Users' Manual 100 .U5753 No. 695 1977 NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, a technical (3) basis for equity in trade, and (4) technical services to pro- mote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research the Institute for Applied Technology, the Institute for Computer Sciences and Technology, the Office for Information Programs, and the Office of Experimental Technology Incentives Program. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consist- ent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essen- tial services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. The Institute consists of the Office of Measurement Services, and the following center and divisions: Applied Mathematics -

Inflationary Big Bang Cosmology and the New Cosmic Background Radiation Findings
Inflationary Big Bang Cosmology and the New Cosmic Background Radiation Findings By Richard M. Todaro American Physical Society June 2001 With special thanks to Dr. Paul L. Richards, Professor of Physics at the University of California, Berkeley for his kind and patient telephone tutorial on Big Bang cosmology. A trio of new findings lends strong support to a powerful idea called “inflation” that explains many of the observed characteristics of our universe. The findings relate to the slight variations in the faint microwave energy that permeates the cosmos. The size and distribution of these variations agree well with the theory of inflation, which holds that the entire universe underwent an incredibly brief period of mind-bogglingly vast expansion (a hyper-charged Big Bang) before slowing down to the slower rate of expansion observed today. Combining the new findings about the cosmic microwave background radiation with inflation, cosmologists (the scientists who study the origin of the cosmos) believe they also have a compelling explanation for why stars and galaxies have their irregular distribution across the observable universe. Some cosmologists now believe that not only is the irregular distribution of “ordinary matter” related to the nature of these variations in the cosmic background radiation, but through inflation, both are related to the conditions within that incredibly dense and hot universe at the instant of its creation. (Such ordinary matter includes the elements that make up our Sun and our Earth and, ultimately, human beings.) Cosmologists have long believed that every bit of matter in the entire observable Universe – all the planets, stars, and galaxies, which we can see through powerful telescopes, as well as all the things we can’t see directly but know must be there due to the effects they produce – was once compressed together into a very dense and very hot arbitrarily small volume. -

Mathematics 3-4
Mathematics 3-4 Mathematics Department Phillips Exeter Academy Exeter, NH August 2021 To the Student Contents: Members of the PEA Mathematics Department have written the material in this book. As you work through it, you will discover that algebra, geometry, and trigonometry have been integrated into a mathematical whole. There is no Chapter 5, nor is there a section on tangents to circles. The curriculum is problem-centered, rather than topic-centered. Techniques and theorems will become apparent as you work through the problems, and you will need to keep appropriate notes for your records | there are no boxes containing important theorems. There is no index as such, but the reference section that starts on page 103 should help you recall the meanings of key words that are defined in the problems (where they usually appear italicized). Problem solving: Approach each problem as an exploration. Reading each question care- fully is essential, especially since definitions, highlighted in italics, are routinely inserted into the problem texts. It is important to make accurate diagrams. Here are a few useful strategies to keep in mind: create an easier problem, use the guess-and-check technique as a starting point, work backwards, recall work on a similar problem. It is important that you work on each problem when assigned, since the questions you may have about a problem will likely motivate class discussion the next day. Problem solving requires persistence as much as it requires ingenuity. When you get stuck, or solve a problem incorrectly, back up and start over. Keep in mind that you're probably not the only one who is stuck, and that may even include your teacher. -

Powers of 10 & Scientific Notation
Physics 151 Powers of 10 & Scientific Notation In all of the physical sciences, we encounter many numbers that are so large or small that it would be exceedingly cumbersome to write them with dozens of trailing or leading zeroes. Since our number system is “base-10” (based on powers of 10), it is far more convenient to write very large and very small numbers in a special exponential notation called scientific notation. In scientific notation, a number is rewritten as a simple decimal multiplied by 10 raised to some power, n, like this: x.xxxx... × 10n Powers of Ten Remember that the powers of 10 are as follows: 0 10 = 1 1 –1 1 10 = 10 10 = 0.1 = 10 2 –2 1 10 = 100 10 = 0.01 = 100 3 –3 1 10 = 1000 10 = 0.001 = 1000 4 –4 1 10 = 10,000 10 = 0.0001 = 10,000 ...and so forth. There are some important powers that we use often: 103 = 1000 = one thousand 10–3 = 0.001 = one thousandth 106 = 1,000,000 = one million 10–6 = 0.000 001 = one millionth 109 = 1,000,000,000 = one billion 10–9 = 0.000 000 001 = one billionth 1012 = 1,000,000,000,000 = one trillion 10–12 = 0.000 000 000 001 = one trillionth In the left-hand columns above, where n is positive, note that n is simply the same as the number of zeroes in the full written-out form of the number! However, in the right-hand columns where n is negative, note that |n| is one greater than the number of place-holding zeroes. -

Radiowave Propagation 1 Introduction Wireless Communication Relies on Electromagnetic Waves to Carry Information from One Point to Another
Lecture 2 Radiowave Propagation 1 Introduction Wireless communication relies on electromagnetic waves to carry information from one point to another. This propagation forms our physical channel. It is essential that we understand it if we are going to be able to understand and design wireless systems. This is a complex subject and will be our focus for several lectures. We will begin by considering the case of free-space propagation. First, however, we need to learn/review the dBm logarithmic power scale, as we will use this extensively. 2 Logarithmic Power Scales Power levels in radio systems can have very large dynamic ranges. For example, a CB (“citizen’s band”) radio might transmit 4W and receive as little as 10 fW ( 10−14 W ). That’s a dynamic range of more than 14 orders of magnitude. Instead of having to use factors like 1014 , it is usually more convenient to work on a logarithmic scale. Also, most of the systems we will consider can be treated as a cascade of blocks in which each block adds a gain factor. Since logarithms turn factors into sums, working on a log scale often simplifies analysis – you add instead of multiply. A dimensionless number A can be represented on a decibel scale as A =10log A dB (1) A 10 A=10 dB / If P is a power level then it has dimensions. Therefore, we cannot directly convert it to a dB scale. But we can get a dimensionless number by taking the ratio of P to some reference power. The most commonly used reference in RF telecom is one milliwatt, and we write dBm, meaning dB relative to a milliwatt. -
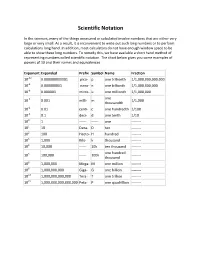
Scientific Notation
Scientific Notation In the sciences, many of the things measured or calculated involve numbers that are either very large or very small. As a result, it is inconvenient to write out such long numbers or to perform calculations long hand. In addition, most calculators do not have enough window space to be able to show these long numbers. To remedy this, we have available a short hand method of representing numbers called scientific notation. The chart below gives you some examples of powers of 10 and their names and equivalences. Exponent Expanded Prefix Symbol Name Fraction 10-12 0.000000000001 pico- p one trillionth 1/1,000,000,000,000 10-9 0.000000001 nano- n one billionth 1/1,000,000,000 10-6 0.000001 micro- u one millionth 1/1,000,000 one 10-3 0.001 milli- m 1/1,000 thousandth 10-2 0.01 centi- c one hundredth 1/100 10-1 0.1 deci- d one tenth 1/10 100 1 ------ ------ one -------- 101 10 Deca- D ten -------- 102 100 Hecto- H hundred -------- 103 1,000 Kilo- k thousand -------- 104 10,000 ------ 10k ten thousand -------- one hundred 105 100,000 ------ 100k -------- thousand 106 1,000,000 Mega- M one million -------- 109 1,000,000,000 Giga- G one billion -------- 1012 1,000,000,000,000 Tera- T one trillion -------- 1015 1,000,000,000,000,000 Peta- P one quadrillion -------- Powers of 10 and Place Value.......... Multiplying by 10, 100, or 1000 in the following problems just means to add the number of zeroes to the number being multiplied. -

Rare-Earth Elements
Rare-Earth Elements Chapter O of Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply Professional Paper 1802–O U.S. Department of the Interior U.S. Geological Survey Periodic Table of Elements 1A 8A 1 2 hydrogen helium 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 lithium beryllium boron carbon nitrogen oxygen fluorine neon 6.94 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 13 14 15 16 17 18 sodium magnesium aluminum silicon phosphorus sulfur chlorine argon 22.99 24.31 3B 4B 5B 6B 7B 8B 11B 12B 26.98 28.09 30.97 32.06 35.45 39.95 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 potassium calcium scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc gallium germanium arsenic selenium bromine krypton 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.39 69.72 72.64 74.92 78.96 79.90 83.79 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 rubidium strontium yttrium zirconium niobium molybdenum technetium ruthenium rhodium palladium silver cadmium indium tin antimony tellurium iodine xenon 85.47 87.62 88.91 91.22 92.91 95.96 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 cesium barium hafnium tantalum tungsten rhenium osmium iridium platinum gold mercury thallium lead bismuth polonium astatine radon 132.9 137.3 178.5 180.9 183.9 186.2 190.2 192.2 195.1 197.0 200.5 204.4 207.2 209.0 (209) (210) (222) 87 88 104 105 106 107 108 109 110 111 112 113 114 115 -
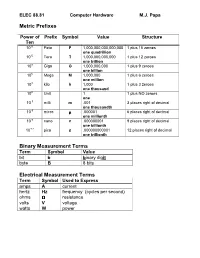
Metric Prefixes Binary Measurement Terms Electrical Measurement Terms
ELEC 88.81 Computer Hardware M.J. Papa Metric Prefixes Power of Prefix Symbol Value Structure Ten 10 15 Peta P 1,000,000,000,000,000 1 plus 15 zeroes one quadrillion 10 12 Tera T 1,000,000,000,000 1 plus 12 zeroes one trillion 10 9 Giga G 1,000,000,000 1 plus 9 zeroes one billion 10 6 Mega M 1,000,000 1 plus 6 zeroes one million 10 3 kilo k 1,000 1 plus 3 zeroes one thousand 10 0 Unit 1 1 plus NO zeroes one 10 -3 milli m .001 3 places right of decimal one thousandth 10 -6 micro µµµ .000001 6 places right of decimal one millionth 10 -9 nano n .000000001 9 places right of decimal one billionth 10 -12 pico p .000000000001 12 places right of decimal one trillionth Binary Measurement Terms Term Symbol Value bit b binary dig it byte B 8 bits Electrical Measurement Terms Term Symbol Used to Express amps A current hertz Hz frequency (cycles per second) ohms ΩΩΩ resistance volts V voltage watts W power ELEC 88.81 Computer Hardware M.J. Papa Converting Units Within the Metric System Using a Multiplication Factor Units can be converted by using a multiplication factor. Use of a multiplication factor results in simply moving the decimal point left or right. Use the table below to convert units. When Moving Left to Right Use Positive Power of Ten 10 12 10 9 10 6 10 3 10 0 10 -3 10 -6 10 -9 10 -12 tera giga mega kilo milli micro nano pico T G M k • m µ n p When Moving Right to Left Use Negative Power of Ten Example: Convert 50 mA to A. -

Quantum Universe
QUANTUM UNIVERSE THE REVOLUTION IN 21ST CENTURY PARTICLE PHYSICS DOE / NSF HIGH ENERGY PHYSICS ADVISORY PANEL QUANTUM UNIVERSE COMMITTEE QUANTUM UNIVERSE THE REVOLUTION IN 21ST CENTURY PARTICLE PHYSICS What does “Quantum Universe” mean? To discover what the universe is made of and how it works is the challenge of particle physics. Quantum Universe presents the quest to explain the universe in terms of quantum physics, which governs the behavior of the microscopic, subatomic world. It describes a revolution in particle physics and a quantum leap in our understanding of the mystery and beauty of the universe. DOE / NSF HIGH ENERGY PHYSICS ADVISORY PANEL QUANTUM UNIVERSE COMMITTEE QUANTUM UNIVERSE COMMITTEE MEMBERS ANDREAS ALBRECHT EDWARD KOLB University of California at Davis Fermilab University of Chicago SAMUEL ARONSON Brookhaven National Laboratory JOSEPH LYKKEN Fermilab KEITH BAKER Hampton University HITOSHI MURAYAMA Thomas Jefferson National Accelerator Facility Institute for Advanced Study, Princeton University of California, Berkeley JONATHAN BAGGER Johns Hopkins University HAMISH ROBERTSON University of Washington NEIL CALDER Stanford Linear Accelerator Center JAMES SIEGRIST Stanford University Lawrence Berkeley National Laboratory University of California, Berkeley PERSIS DRELL, CHAIR Stanford Linear Accelerator Center SIMON SWORDY Stanford University University of Chicago EVALYN GATES JOHN WOMERSLEY University of Chicago Fermilab FRED GILMAN Carnegie Mellon University JUDITH JACKSON Fermilab STEVEN KAHN Physics Department, Stanford -

Time and Frequency Users' Manual
1 United States Department of Commerce 1 j National Institute of Standards and Technology NAT L. INST. OF STAND & TECH R.I.C. NIST PUBLICATIONS I A111D3 MSDD3M NIST Special Publication 559 (Revised 1990) Time and Frequency Users Manual George Kamas Michael A. Lombardi NATIONAL INSTITUTE OF STANDARDS & TECHNOLOGY Research Information Center Gakhersburg, MD 20699 NIST Special Publication 559 (Revised 1990) Time and Frequency Users Manual George Kamas Michael A. Lombardi Time and Frequency Division Center for Atomic, Molecular, and Optical Physics National Measurement Laboratory National Institute of Standards and Technology Boulder, Colorado 80303-3328 (Supersedes NBS Special Publication 559 dated November 1979) September 1990 U.S. Department of Commerce Robert A. Mosbacher, Secretary National Institute of Standards and Technology John W. Lyons, Director National Institute of Standards U.S. Government Printing Office For sale by the Superintendent and Technology Washington: 1990 of Documents Special Publication 559 (Rev. 1990) U.S. Government Printing Office Natl. Inst. Stand. Technol. Washington, DC 20402 Spec. Publ. 559 (Rev. 1990) 160 pages (Sept. 1990) CODEN: NSPUE2 ABSTRACT This book is for the person who needs information about making time and frequency measurements. It is written at a level that will satisfy those with a casual interest as well as laboratory engineers and technicians who use time and frequency every day. It includes a brief discussion of time scales, discusses the roles of the National Institute of Standards and Technology (NIST) and other national laboratories, and explains how time and frequency are internationally coordinated. It also describes the available time and frequency services and how to use them. -
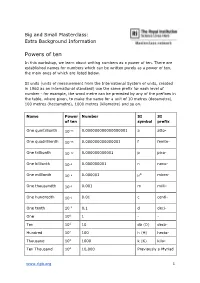
Powers of Ten
Big and Small Masterclass: Extra Background Information Powers of ten In this workshop, we learn about writing numbers as a power of ten. There are established names for numbers which can be written purely as a power of ten, the main ones of which are listed below. SI units (units of measurement from the International System of units, created in 1960 as an international standard) use the same prefix for each level of number - for example, the word metre can be preceded by any of the prefixes in the table, where given, to make the name for a unit of 10 metres (decametre), 100 metres (hectometre), 1000 metres (kilometre) and so on. Name Power Number SI SI of ten symbol prefix One quintillionth 10−18 0.000000000000000001 a atto- One quadrillionth 10−15 0.000000000000001 f femto- One trillionth 10−12 0.000000000001 p pico- One billionth 10−9 0.000000001 n nano- One millionth 10−6 0.000001 µ* micro- One thousandth 10−3 0.001 m milli- One hundredth 10−2 0.01 c centi- One tenth 10-1 0.1 d deci- One 100 1 - - Ten 101 10 da (D) deca- Hundred 102 100 h (H) hecto- Thousand 103 1000 k (K) kilo- Ten Thousand 104 10,000 Previously a Myriad www.rigb.org 1 100 Thousand 105 100,000 Previously a Lakh Million 106 1,000,000 M mega- Ten Million 107 10,000,000 Previously a Crore Billion 109 1,000,000,000 G giga- Trillion 1012 1,000,000,000,000 T tera- Quadrillion 1015 1,000,000,000,000,000 P peta- Quintillion 1018 1,000,000,000,000,000,000 E exa- Sextillion 2021 1,000,000,000,000,000,000,000 Z zetta- Septillion 1024 1,000,000,000,000,000,000,000,000 Y yotta- Octillion 1027 1,000,000,000,000,000,000,000,000,000 B bronta- * This is the Greek letter mu, used to denote ‘micro’, as ‘m’ was already taken.