Powers of 10 & Scientific Notation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TI 83/84: Scientific Notation on Your Calculator
TI 83/84: Scientific Notation on your calculator this is above the comma, next to the square root! choose the proper MODE : Normal or Sci entering numbers: 2.39 x 106 on calculator: 2.39 2nd EE 6 ENTER reading numbers: 2.39E6 on the calculator means for us. • When you're in Normal mode, the calculator will write regular numbers unless they get too big or too small, when it will switch to scientific notation. • In Sci mode, the calculator displays every answer as scientific notation. • In both modes, you can type in numbers in scientific notation or as regular numbers. Humans should never, ever, ever write scientific notation using the calculator’s E notation! Try these problems. Answer in scientific notation, and round decimals to two places. 2.39 x 1016 (5) 2.39 x 109+ 4.7 x 10 10 (4) 4.7 x 10−3 3.01 103 1.07 10 0 2.39 10 5 4.94 10 10 5.09 1018 − 3.76 10−− 1 2.39 10 5 7.93 10 8 Remember to change your MODE back to Normal when you're done. Using the STORE key: Let's say that you want to store a number so that you can use it later, or that you want to store answers for several different variables, and then use them together in one problem. Here's how: Enter the number, then press STO (above the ON key), then press ALPHA and the letter you want. (The letters are in alphabetical order above the other keys.) Then press ENTER. -

Stage 1: the Number Sequence
1 stage 1: the number sequence Big Idea: Quantity Quantity is the big idea that describes amounts, or sizes. It is a fundamental idea that refers to quantitative properties; the size of things (magnitude), and the number of things (multitude). Why is Quantity Important? Quantity means that numbers represent amounts. If students do not possess an understanding of Quantity, their knowledge of foundational mathematics will be undermined. Understanding Quantity helps students develop number conceptualization. In or- der for children to understand quantity, they need foundational experiences with counting, identifying numbers, sequencing, and comparing. Counting, and using numerals to quantify collections, form the developmental progression of experiences in Stage 1. Children who understand number concepts know that numbers are used to describe quantities and relationships’ between quantities. For example, the sequence of numbers is determined by each number’s magnitude, a concept that not all children understand. Without this underpinning of understanding, a child may perform rote responses, which will not stand the test of further, rigorous application. The developmental progression of experiences in Stage 1 help students actively grow a strong number knowledge base. Stage 1 Learning Progression Concept Standard Example Description Children complete short sequences of visual displays of quantities beginning with 1. Subsequently, the sequence shows gaps which the students need to fill in. The sequencing 1.1: Sequencing K.CC.2 1, 2, 3, 4, ? tasks ask students to show that they have quantity and number names in order of magnitude and to associate quantities with numerals. 1.2: Identifying Find the Students see the visual tool with a numeral beneath it. -

Using Concrete Scales: a Practical Framework for Effective Visual Depiction of Complex Measures Fanny Chevalier, Romain Vuillemot, Guia Gali
Using Concrete Scales: A Practical Framework for Effective Visual Depiction of Complex Measures Fanny Chevalier, Romain Vuillemot, Guia Gali To cite this version: Fanny Chevalier, Romain Vuillemot, Guia Gali. Using Concrete Scales: A Practical Framework for Effective Visual Depiction of Complex Measures. IEEE Transactions on Visualization and Computer Graphics, Institute of Electrical and Electronics Engineers, 2013, 19 (12), pp.2426-2435. 10.1109/TVCG.2013.210. hal-00851733v1 HAL Id: hal-00851733 https://hal.inria.fr/hal-00851733v1 Submitted on 8 Jan 2014 (v1), last revised 8 Jan 2014 (v2) HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Using Concrete Scales: A Practical Framework for Effective Visual Depiction of Complex Measures Fanny Chevalier, Romain Vuillemot, and Guia Gali a b c Fig. 1. Illustrates popular representations of complex measures: (a) US Debt (Oto Godfrey, Demonocracy.info, 2011) explains the gravity of a 115 trillion dollar debt by progressively stacking 100 dollar bills next to familiar objects like an average-sized human, sports fields, or iconic New York city buildings [15] (b) Sugar stacks (adapted from SugarStacks.com) compares caloric counts contained in various foods and drinks using sugar cubes [32] and (c) How much water is on Earth? (Jack Cook, Woods Hole Oceanographic Institution and Howard Perlman, USGS, 2010) shows the volume of oceans and rivers as spheres whose sizes can be compared to that of Earth [38]. -

Large but Finite
Department of Mathematics MathClub@WMU Michigan Epsilon Chapter of Pi Mu Epsilon Large but Finite Drake Olejniczak Department of Mathematics, WMU What is the biggest number you can imagine? Did you think of a million? a billion? a septillion? Perhaps you remembered back to the time you heard the term googol or its show-off of an older brother googolplex. Or maybe you thought of infinity. No. Surely, `infinity' is cheating. Large, finite numbers, truly monstrous numbers, arise in several areas of mathematics as well as in astronomy and computer science. For instance, Euclid defined perfect numbers as positive integers that are equal to the sum of their proper divisors. He is also credited with the discovery of the first four perfect numbers: 6, 28, 496, 8128. It may be surprising to hear that the ninth perfect number already has 37 digits, or that the thirteenth perfect number vastly exceeds the number of particles in the observable universe. However, the sequence of perfect numbers pales in comparison to some other sequences in terms of growth rate. In this talk, we will explore examples of large numbers such as Graham's number, Rayo's number, and the limits of the universe. As well, we will encounter some fast-growing sequences and functions such as the TREE sequence, the busy beaver function, and the Ackermann function. Through this, we will uncover a structure on which to compare these concepts and, hopefully, gain a sense of what it means for a number to be truly large. 4 p.m. Friday October 12 6625 Everett Tower, WMU Main Campus All are welcome! http://www.wmich.edu/mathclub/ Questions? Contact Patrick Bennett ([email protected]) . -

Decimal Rounding
Mathematics Instructional Plan – Grade 5 Decimal Rounding Strand: Number and Number Sense Topic: Rounding decimals, through the thousandths, to the nearest whole number, tenth, or hundredth. Primary SOL: 5.1 The student, given a decimal through thousandths, will round to the nearest whole number, tenth, or hundredth. Materials Base-10 blocks Decimal Rounding activity sheet (attached) “Highest or Lowest” Game (attached) Open Number Lines activity sheet (attached) Ten-sided number generators with digits 0–9, or decks of cards Vocabulary approximate, between, closer to, decimal number, decimal point, hundredth, rounding, tenth, thousandth, whole Student/Teacher Actions: What should students be doing? What should teachers be doing? 1. Begin with a review of decimal place value: Display a decimal in the thousandths place, such as 34.726, using base-10 blocks. In pairs, have students discuss how to read the number using place-value names, and review the decimal place each digit holds. Briefly have students share their thoughts with the class by asking: What digit is in the tenths place? The hundredths place? The thousandths place? Who would like to share how to say this decimal using place value? 2. Ask, “If this were a monetary amount, $34.726, how would we determine the amount to the nearest cent?” Allow partners or small groups to discuss this question. It may be helpful if students underlined the place that indicates cents (the hundredths place) in order to keep track of the rounding place. Distribute the Open Number Lines activity sheet and display this number line on the board. Guide students to recall that the nearest cent is the same as the nearest hundredth of a dollar. -

Primitive Number Types
Primitive number types Values of each of the primitive number types Java has these 4 primitive integral types: byte: A value occupies 1 byte (8 bits). The range of values is -2^7..2^7-1, or -128..127 short: A value occupies 2 bytes (16 bits). The range of values is -2^15..2^15-1 int: A value occupies 4 bytes (32 bits). The range of values is -2^31..2^31-1 long: A value occupies 8 bytes (64 bits). The range of values is -2^63..2^63-1 and two “floating-point” types, whose values are approximations to the real numbers: float: A value occupies 4 bytes (32 bits). double: A value occupies 8 bytes (64 bits). Values of the integral types are maintained in two’s complement notation (see the dictionary entry for two’s complement notation). A discussion of floating-point values is outside the scope of this website, except to say that some bits are used for the mantissa and some for the exponent and that infinity and NaN (not a number) are both floating-point values. We don’t discuss this further. Generally, one uses mainly types int and double. But if you are declaring a large array and you know that the values fit in a byte, you can save ¾ of the space using a byte array instead of an int array, e.g. byte[] b= new byte[1000]; Operations on the primitive integer types Types byte and short have no operations. Instead, operations on their values int and long operations are treated as if the values were of type int. -

Grade 7/8 Math Circles the Scale of Numbers Introduction
Faculty of Mathematics Centre for Education in Waterloo, Ontario N2L 3G1 Mathematics and Computing Grade 7/8 Math Circles November 21/22/23, 2017 The Scale of Numbers Introduction Last week we quickly took a look at scientific notation, which is one way we can write down really big numbers. We can also use scientific notation to write very small numbers. 1 × 103 = 1; 000 1 × 102 = 100 1 × 101 = 10 1 × 100 = 1 1 × 10−1 = 0:1 1 × 10−2 = 0:01 1 × 10−3 = 0:001 As you can see above, every time the value of the exponent decreases, the number gets smaller by a factor of 10. This pattern continues even into negative exponent values! Another way of picturing negative exponents is as a division by a positive exponent. 1 10−6 = = 0:000001 106 In this lesson we will be looking at some famous, interesting, or important small numbers, and begin slowly working our way up to the biggest numbers ever used in mathematics! Obviously we can come up with any arbitrary number that is either extremely small or extremely large, but the purpose of this lesson is to only look at numbers with some kind of mathematical or scientific significance. 1 Extremely Small Numbers 1. Zero • Zero or `0' is the number that represents nothingness. It is the number with the smallest magnitude. • Zero only began being used as a number around the year 500. Before this, ancient mathematicians struggled with the concept of `nothing' being `something'. 2. Planck's Constant This is the smallest number that we will be looking at today other than zero. -

Basic Statistics Range, Mean, Median, Mode, Variance, Standard Deviation
Kirkup Chapter 1 Lecture Notes SEF Workshop presentation = Science Fair Data Analysis_Sohl.pptx EDA = Exploratory Data Analysis Aim of an Experiment = Experiments should have a goal. Make note of interesting issues for later. Be careful not to get side-tracked. Designing an Experiment = Put effort where it makes sense, do preliminary fractional error analysis to determine where you need to improve. Units analysis and equation checking Units website Writing down values: scientific notation and SI prefixes http://en.wikipedia.org/wiki/Metric_prefix Significant Figures Histograms in Excel Bin size – reasonable round numbers 15-16 not 14.8-16.1 Bin size – reasonable quantity: Rule of Thumb = # bins = √ where n is the number of data points. Basic Statistics range, mean, median, mode, variance, standard deviation Population, Sample Cool trick for small sample sizes. Estimate this works for n<12 or so. √ Random Error vs. Systematic Error Metric prefixes m n [n 1] Prefix Symbol 1000 10 Decimal Short scale Long scale Since 8 24 yotta Y 1000 10 1000000000000000000000000septillion quadrillion 1991 7 21 zetta Z 1000 10 1000000000000000000000sextillion trilliard 1991 6 18 exa E 1000 10 1000000000000000000quintillion trillion 1975 5 15 peta P 1000 10 1000000000000000quadrillion billiard 1975 4 12 tera T 1000 10 1000000000000trillion billion 1960 3 9 giga G 1000 10 1000000000billion milliard 1960 2 6 mega M 1000 10 1000000 million 1960 1 3 kilo k 1000 10 1000 thousand 1795 2/3 2 hecto h 1000 10 100 hundred 1795 1/3 1 deca da 1000 10 10 ten 1795 0 0 1000 -

Arithmetic Algorisms in Different Bases
Arithmetic Algorisms in Different Bases The Addition Algorithm Addition Algorithm - continued To add in any base – Step 1 14 Step 2 1 Arithmetic Algorithms in – Add the digits in the “ones ”column to • Add the digits in the “base” 14 + 32 5 find the number of “1s”. column. + 32 2 + 4 = 6 5 Different Bases • If the number is less than the base place – If the sum is less than the base 1 the number under the right hand column. 6 > 5 enter under the “base” column. • If the number is greater than or equal to – If the sum is greater than or equal 1+1+3 = 5 the base, express the number as a base 6 = 11 to the base express the sum as a Addition, Subtraction, 5 5 = 10 5 numeral. The first digit indicates the base numeral. Carry the first digit Multiplication and Division number of the base in the sum so carry Place 1 under to the “base squared” column and Carry the 1 five to the that digit to the “base” column and write 4 + 2 and carry place the second digit under the 52 column. the second digit under the right hand the 1 five to the “base” column. Place 0 under the column. “fives” column Step 3 “five” column • Repeat this process to the end. The sum is 101 5 Text Chapter 4 – Section 4 No in-class assignment problem In-class Assignment 20 - 1 Example of Addition The Subtraction Algorithm A Subtraction Problem 4 9 352 Find: 1 • 3 + 2 = 5 < 6 →no Subtraction in any base, b 35 2 • 2−4 can not do. -

A Child Thinking About Infinity
A Child Thinking About Infinity David Tall Mathematics Education Research Centre University of Warwick COVENTRY CV4 7AL Young children’s thinking about infinity can be fascinating stories of extrapolation and imagination. To capture the development of an individual’s thinking requires being in the right place at the right time. When my youngest son Nic (then aged seven) spoke to me for the first time about infinity, I was fortunate to be able to tape-record the conversation for later reflection on what was happening. It proved to be a fascinating document in which he first treated infinity as a very large number and used his intuitions to think about various arithmetic operations on infinity. He also happened to know about “minus numbers” from earlier experiences with temperatures in centigrade. It was thus possible to ask him not only about arithmetic with infinity, but also about “minus infinity”. The responses were thought-provoking and amazing in their coherent relationships to his other knowledge. My research in studying infinite concepts in older students showed me that their ideas were influenced by their prior experiences. Almost always the notion of “limit” in some dynamic sense was met before the notion of one to one correspondences between infinite sets. Thus notions of “variable size” had become part of their intuition that clashed with the notion of infinite cardinals. For instance, Tall (1980) reported a student who considered that the limit of n2/n! was zero because the top is a “smaller infinity” than the bottom. It suddenly occurred to me that perhaps I could introduce Nic to the concept of cardinal infinity to see what this did to his intuitions. -
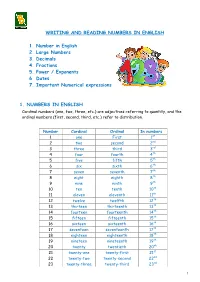
WRITING and READING NUMBERS in ENGLISH 1. Number in English 2
WRITING AND READING NUMBERS IN ENGLISH 1. Number in English 2. Large Numbers 3. Decimals 4. Fractions 5. Power / Exponents 6. Dates 7. Important Numerical expressions 1. NUMBERS IN ENGLISH Cardinal numbers (one, two, three, etc.) are adjectives referring to quantity, and the ordinal numbers (first, second, third, etc.) refer to distribution. Number Cardinal Ordinal In numbers 1 one First 1st 2 two second 2nd 3 three third 3rd 4 four fourth 4th 5 five fifth 5th 6 six sixth 6th 7 seven seventh 7th 8 eight eighth 8th 9 nine ninth 9th 10 ten tenth 10th 11 eleven eleventh 11th 12 twelve twelfth 12th 13 thirteen thirteenth 13th 14 fourteen fourteenth 14th 15 fifteen fifteenth 15th 16 sixteen sixteenth 16th 17 seventeen seventeenth 17th 18 eighteen eighteenth 18th 19 nineteen nineteenth 19th 20 twenty twentieth 20th 21 twenty-one twenty-first 21st 22 twenty-two twenty-second 22nd 23 twenty-three twenty-third 23rd 1 24 twenty-four twenty-fourth 24th 25 twenty-five twenty-fifth 25th 26 twenty-six twenty-sixth 26th 27 twenty-seven twenty-seventh 27th 28 twenty-eight twenty-eighth 28th 29 twenty-nine twenty-ninth 29th 30 thirty thirtieth 30th st 31 thirty-one thirty-first 31 40 forty fortieth 40th 50 fifty fiftieth 50th 60 sixty sixtieth 60th th 70 seventy seventieth 70 th 80 eighty eightieth 80 90 ninety ninetieth 90th 100 one hundred hundredth 100th 500 five hundred five hundredth 500th 1,000 One/ a thousandth 1000th thousand one thousand one thousand five 1500th 1,500 five hundred, hundredth or fifteen hundred 100,000 one hundred hundred thousandth 100,000th thousand 1,000,000 one million millionth 1,000,000 ◊◊ Click on the links below to practice your numbers: http://www.manythings.org/wbg/numbers-jw.html https://www.englisch-hilfen.de/en/exercises/numbers/index.php 2 We don't normally write numbers with words, but it's possible to do this. -

IEEE Standard 754 for Binary Floating-Point Arithmetic
Work in Progress: Lecture Notes on the Status of IEEE 754 October 1, 1997 3:36 am Lecture Notes on the Status of IEEE Standard 754 for Binary Floating-Point Arithmetic Prof. W. Kahan Elect. Eng. & Computer Science University of California Berkeley CA 94720-1776 Introduction: Twenty years ago anarchy threatened floating-point arithmetic. Over a dozen commercially significant arithmetics boasted diverse wordsizes, precisions, rounding procedures and over/underflow behaviors, and more were in the works. “Portable” software intended to reconcile that numerical diversity had become unbearably costly to develop. Thirteen years ago, when IEEE 754 became official, major microprocessor manufacturers had already adopted it despite the challenge it posed to implementors. With unprecedented altruism, hardware designers had risen to its challenge in the belief that they would ease and encourage a vast burgeoning of numerical software. They did succeed to a considerable extent. Anyway, rounding anomalies that preoccupied all of us in the 1970s afflict only CRAY X-MPs — J90s now. Now atrophy threatens features of IEEE 754 caught in a vicious circle: Those features lack support in programming languages and compilers, so those features are mishandled and/or practically unusable, so those features are little known and less in demand, and so those features lack support in programming languages and compilers. To help break that circle, those features are discussed in these notes under the following headings: Representable Numbers, Normal and Subnormal, Infinite