Information to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clusters – Contemporary Insight in Structure and Bonding 174 Structure and Bonding
Structure and Bonding 174 Series Editor: D.M.P. Mingos Stefanie Dehnen Editor Clusters – Contemporary Insight in Structure and Bonding 174 Structure and Bonding Series Editor: D.M.P. Mingos, Oxford, United Kingdom Editorial Board: X. Duan, Beijing, China L.H. Gade, Heidelberg, Germany Y. Lu, Urbana, IL, USA F. Neese, Mulheim€ an der Ruhr, Germany J.P. Pariente, Madrid, Spain S. Schneider, Gottingen,€ Germany D. Stalke, Go¨ttingen, Germany Aims and Scope Structure and Bonding is a publication which uniquely bridges the journal and book format. Organized into topical volumes, the series publishes in depth and critical reviews on all topics concerning structure and bonding. With over 50 years of history, the series has developed from covering theoretical methods for simple molecules to more complex systems. Topics addressed in the series now include the design and engineering of molecular solids such as molecular machines, surfaces, two dimensional materials, metal clusters and supramolecular species based either on complementary hydrogen bonding networks or metal coordination centers in metal-organic framework mate- rials (MOFs). Also of interest is the study of reaction coordinates of organometallic transformations and catalytic processes, and the electronic properties of metal ions involved in important biochemical enzymatic reactions. Volumes on physical and spectroscopic techniques used to provide insights into structural and bonding problems, as well as experimental studies associated with the development of bonding models, reactivity pathways and rates of chemical processes are also relevant for the series. Structure and Bonding is able to contribute to the challenges of communicating the enormous amount of data now produced in contemporary research by producing volumes which summarize important developments in selected areas of current interest and provide the conceptual framework necessary to use and interpret mega- databases. -

Covalent and Noncovalent Intermediates of an NAD Utilizing Enzyme, Human CD38
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Chemistry & Biology Article Covalent and Noncovalent Intermediates of an NAD Utilizing Enzyme, Human CD38 Qun Liu,1 Irina A. Kriksunov,1 Hong Jiang,2 Richard Graeff,4 Hening Lin,2 Hon Cheung Lee,4,5,* and Quan Hao1,3,5,* 1MacCHESS, Cornell High Energy Synchrotron Source 2Department of Chemistry and Chemical Biology 3School of Applied & Engineering Physics Cornell University, Ithaca, NY 14853, USA 4Department of Pharmacology, University of Minnesota, Minneapolis, MN 55455, USA 5Department of Physiology, University of Hong Kong, Hong Kong, China *Correspondence: [email protected] (H.C.L.), [email protected] (Q.H.) DOI 10.1016/j.chembiol.2008.08.007 SUMMARY These processes are known to have important cellular and physiological functions in DNA repair (Lombard et al., 2005; Enzymatic utilization of nicotinamide adenine dinu- Michan and Sinclair, 2007), transcriptional regulation (Blander cleotide (NAD) has increasingly been shown to have and Guarente, 2004), cellular differentiation and proliferation, fundamental roles in gene regulation, signal trans- aging (Hassa et al., 2006), and calcium signaling (Lee, 2001; duction, and protein modification. Many of the pro- Lee et al., 1999). cesses require the cleavage of the nicotinamide moi- Although NAD is a substrate for multiple enzymes, the initial ety from the substrate and the formation of a reactive steps of the cleavage and release of the nicotinamide moiety are conserved. The nature of the subsequent intermediates intermediate. Using X-ray crystallography, we show formed, on the other hand, has been a widely debatable issue. -

Planar Cyclopenten‐4‐Yl Cations: Highly Delocalized Π Aromatics
Angewandte Research Articles Chemie How to cite: Angew.Chem. Int. Ed. 2020, 59,18809–18815 Carbocations International Edition: doi.org/10.1002/anie.202009644 German Edition: doi.org/10.1002/ange.202009644 Planar Cyclopenten-4-yl Cations:Highly Delocalized p Aromatics Stabilized by Hyperconjugation Samuel Nees,Thomas Kupfer,Alexander Hofmann, and Holger Braunschweig* 1 B Abstract: Theoretical studies predicted the planar cyclopenten- being energetically favored by 18.8 kcalmolÀ over 1 (MP3/ 4-yl cation to be aclassical carbocation, and the highest-energy 6-31G**).[11–13] Thebishomoaromatic structure 1B itself is + 1 isomer of C5H7 .Hence,its existence has not been verified about 6–14 kcalmolÀ lower in energy (depending on the level experimentally so far.Wewere now able to isolate two stable of theory) than the classical planar structure 1C,making the derivatives of the cyclopenten-4-yl cation by reaction of bulky cyclopenten-4-yl cation (1C)the least favorable isomer.Early R alanes Cp AlBr2 with AlBr3.Elucidation of their (electronic) solvolysis studies are consistent with these findings,with structures by X-raydiffraction and quantum chemistry studies allylic 1A being the only observable isomer, notwithstanding revealed planar geometries and strong hyperconjugation the nature of the studied cyclopenteneprecursor.[14–18] Thus, interactions primarily from the C Al s bonds to the empty p attempts to generate isomer 1C,orits homoaromatic analog À orbital of the cationic sp2 carbon center.Aclose inspection of 1B,bysolvolysis of 4-Br/OTs-cyclopentene -

Recommending Hartree-Fock Theory with London- Dispersion and Basis
Recommending Hartree-Fock Theory with London- Dispersion and Basis-Set-Superposition Corrections for the Optimization or Quantum Refinement of Protein Structures Lars Goerigk,a* Charles A. Collyer,b Jeffrey R. Reimersc,d* a School of Chemistry, The University of Melbourne, Parkville, Victoria 3010, Australia b School of Molecular Bioscience, The University of Sydney, Sydney, New South Wales 2006, Australia c School of Physics and Advanced Materials, The University of Technology, Sydney, NSW 2007, Australia d Centre for Quantum and Molecular Structure, College of Sciences, Shanghai University, Shanghai 200444, China 1 ABSTRACT We demonstrate the importance of properly accounting for London-dispersion and basis-set superposition-error (BSSE) in quantum-chemical optimizations of protein structures, factors that are often still neglected in contemporary applications. We optimize a portion of an ensemble of conformationally flexible lysozyme structures obtained from highly accurate X-ray crystallography data that serves as a reliable benchmark. We not only analyze root-mean-square deviations from the experimental Cartesian coordinates, but, for the first time, also demonstrate how London-dispersion and BSSE influence crystallographic R factors. Our conclusions parallel recent recommendations for the optimization of small gas-phase peptide structures made by some of the present authors: Hartree-Fock theory extended with Grimme’s recent dispersion and BSSE corrections (HF-D3-gCP) is superior to popular density-functional-theory (DFT) approaches. Not only are statistical errors on average lower with HF-D3-gCP, but also its convergence behavior is much better. In particular, we show that the BP86/6-31G* approach should not be relied upon as a black-box method, despite its widespread use, as its success is based on an unpredictable cancellation of errors. -

An Analysis of Electrophilic Aromatic Substitution: a “Complex Approach” PCCP
Volume 23 Number 9 7 March 2021 Pages 5033–5682 PCCP Physical Chemistry Chemical Physics rsc.li/pccp ISSN 1463-9076 PERSPECTIVE Janez Cerkovnik et al . An analysis of electrophilic aromatic substitution: a “complex approach” PCCP View Article Online PERSPECTIVE View Journal | View Issue An analysis of electrophilic aromatic substitution: a ‘‘complex approach’’† Cite this: Phys. Chem. Chem. Phys., a a b 2021, 23, 5051 Nikola Stamenkovic´, Natasˇa Poklar Ulrih and Janez Cerkovnik * Electrophilic aromatic substitution (EAS) is one of the most widely researched transforms in synthetic organic chemistry. Numerous studies have been carried out to provide an understanding of the nature of its reactivity pattern. There is now a need for a concise and general, but detailed and up-to-date, overview. The basic principles behind EAS are essential to our understanding of what the mechanisms underlying EAS are. To date, textbook overviews of EAS have provided little information about the Received 5th October 2020, mechanistic pathways and chemical species involved. In this review, the aim is to gather and present the Accepted 21st December 2020 up-to-date information relating to reactivity in EAS, with the implication that some of the key concepts DOI: 10.1039/d0cp05245k will be discussed in a scientifically concise manner. In addition, the information presented herein suggests certain new possibilities to advance EAS theory, with particular emphasis on the role of modern Creative Commons Attribution-NonCommercial 3.0 Unported Licence. rsc.li/pccp -

Edinburgh Research Explorer
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Edinburgh Research Explorer Edinburgh Research Explorer Organometallic Neptunium Chemistry Citation for published version: Arnold, P, Dutkiewicz, MS & Walter, O 2017, 'Organometallic Neptunium Chemistry', Chemical Reviews. https://doi.org/10.1021/acs.chemrev.7b00192 Digital Object Identifier (DOI): 10.1021/acs.chemrev.7b00192 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Chemical Reviews General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 11. May. 2020 Organometallic Neptunium Chemistry Polly L. Arnold,*a Michał S. Dutkiewicz,a,b Olaf Walter,b [a] EaStCHEM School of Chemistry, University of Edinburgh, The King’s Buildings, Edinburgh, EH9 3FJ, UK. E-mail: [email protected]. [b] European Commission, DG Joint Research Centre, Directorate G - Nuclear Safety and Security, Advanced Nuclear Knowledge – G.I.5, Postfach 2340, D-76125, Karlsruhe, Germany. ABSTRACT Fifty years have passed since the foundation of organometallic neptunium chemistry, and yet only a handful of complexes have been reported, and even fewer fully characterised. -

Mechanical Keyswitch B3F
Mechanical Keyswitch B3F Miniature, Space-Saving Keyswitch Provides Long Service Life and Easy Mounting ■ Extended mechanical/electrical service life: 10 x 106 operations for 12 x 12 mm type and 1 x 106 operations for the 6 x 6 mm type ■ Ideal for applications such as audio, office and communications equipment, measuring instruments, TVs, VCRs, etc. ■ Taped radial type, vertical type, high force type, and gold-plated contact type are available as series versions ■ Flux-tight base structure allows automatic soldering of the keyswitches onto a PC board Ordering Information Flat Projected ■ B3F-1■■■, B3F-3■■■ 6 x 6 mm type Part Number Switch Without ground terminal With ground terminal Type Plunger height x pitch Operating Force Bags Sticks* Bags Sticks* Standard Flat 4.3 x 6.5 mm General-purpose: 100 g B3F-1000 B3F-1000S B3F-1100 B3F-1100S 150 g B3F-1002 B3F-1002S B3F-1102 B3F-1102S High-force: 260 g B3F-1005 B3F-1005S B3F-1105 B3F-1105S 5.0 x 6.5 mm General-purpose: 100 g B3F-1020 B3F-1020S B3F-1120 B3F-1120S 150 g B3F-1022 B3F-1022S B3F-1122 B3F-1122S High-force: 260 g B3F-1025 B3F-1025S B3F-1125 B3F-1125S 5.0 x 7.5 mm General-purpose: 100 g — — B3F-1110 — Projected 7.3 x 6.5 mm General-purpose: 100 g B3F-1050 B3F-1050S B3F-1150 B3F-1150S 150 g B3F-1052 B3F-1052S B3F-1152 B3F-1152S High-force: 260 g B3F-1055 B3F-1055S B3F-1155 B3F-1155S Vertical Flat 3.15 mm General-purpose: 100 g — — B3F-3100 — 150 g — — B3F-3102 — High-force: 260 g — — B3F-3105 — 3.85 mm General-purpose: 100 g — — B3F-3120 — 150 g — — B3F-3122 — High-force: 260 g — — B3F-3125 — Projected 6.15 mm General-purpose: 100 g — — B3F-3150 — 150 g — — B3F-3152 — High-force: 260 g — — B3F-3155 — * Number of switches per stick: Without ground terminal ... -

Herefore, Incentives Typically Offered and Used for Development Would Be Replaced with the EB-5 Investment
Revised – 9/18/17 CITY OF YPSILANTI REGULAR COUNCIL MEETING CITY COUNCIL CHAMBERS – ONE SOUTH HURON ST. YPSILANTI, MI 48197 TUESDAY, SEPTEMBER 19, 2017 7:00 p.m. I. CALL TO ORDER – II. ROLL CALL – Council Member Bashert P A Council Member Robb P A Mayor Pro-Tem Brown P A Council Member Vogt P A Council Member Murdock P A Mayor Edmonds P A Council Member Richardson P A III. INVOCATION – IV. PLEDGE OF ALLEGIANCE – “I pledge allegiance to the flag, of the United States of America, and to the Republic for which it stands, one nation, under God, indivisible, with liberty and justice for all.” V. AGENDA APPROVAL – VI. INTRODUCTIONS – VII. AUDIENCE PARTICIPATION – VIII. REMARKS BY THE MAYOR – IX. PUBLIC HEARING – International Village - Water Street A. Resolution No. 2017-208, approving purchase agreement. B. Open public hearing C. Resolution No. 2017-209, close public hearing X. PRESENTATIONS – Discussion of Easement Agreement with Michigan Advocacy Program on 15 N. Washington. XI. ORDINANCES – FIRST READING – Ordinance No. 1294 An ordinance to rezone 75 Catherine from Core Neighborhood to Production, Manufacturing & Distribution. A. Resolution No. 2017-210, determination B. Open public hearing C. Resolution No. 2017-211, close public hearing 1 XII. CONSENT AGENDA – Resolution No. 2017-212 1. Resolution No. 2017–213, approving minutes of August 22 and September 5, 2017. 2. Resolution No. 2017-214, approving the issuance of a blanket permit for window signs of any size for the month of October for businesses that participate with the Eastern Michigan University’s “Follow the Green & White Road” homecoming spirit project. -

1 5. Chemical Bonding
5. Chemical Bonding: The Covalent Bond Model 5.1 The Covalent Bond Model Almost all chemical substances are found as aggregates of atoms in the form of molecules and ions produced through the reactions of various atoms of elements except the noble-gas elements which are stable mono-atomic gases. Chemical bond is a term that describes the attractive force that is holding the atoms of the same or different kind of atoms in forming a molecule or ionic solid that has more stability than the individual atoms. Depending on the kinds of atoms participating in the interaction there seem to be three types of bonding: Gaining or Losing Electrons: Ionic bonding: Formed between many ions formed by metal and nonmetallic elements. Sharing Electrons: Covalent bonding: sharing of electrons between two atoms of non-metals. Metallic Bonding: sharing of electrons between many atoms of metals. Ionic Compounds Covalent Compounds Metallic Compounds 1. Metal and non-meal Non-metal and non-meal Metal of one type or, element combinations. elements combinations. combinations of two or metal elements combinations. 2. High melting brittle Gases, liquids, or waxy, low Conducting, high melting, crystalline solids. melting soft solids. malleable, ductile crystalline solids. 3. Do not conduct as a solid Do not conduct electricity at Conduct electricity at solid but conducts electricity any state. and molten states. when molten. 4. Dissolved in water produce Most are soluble in non-polar Insoluble in any type of conducting solutions solvents and few in water. solvents. (electrolytes) and few These solutions are non- are soluble in non-polar conducting (non- solvents. -

Chemical Forces Understanding the Relative Melting/Boiling Points of Two
Chapter 8 – Chemical Forces Understanding the relative melting/boiling points of two substances requires an understanding of the forces acting between molecules of those substances. These intermolecular forces are important for many additional reasons. For example, solubility and vapor pressure are governed by intermolecular forces. The same factors that give rise to intermolecular forces (e.g. bond polarity) can also have a profound impact on chemical reactivity. Chemical Forces Internuclear Distances and Atomic Radii There are four general methods of discussing interatomic distances: van der Waal’s, ionic, covalent, and metallic radii. We will discuss the first three in this section. Each has a unique perspective of the nature of the interaction between interacting atoms/ions. Van der Waal's Radii - half the distance between two nuclei of the same element in the solid state not chemically bonded together (e.g. solid noble gases). In general, the distance of separation between adjacent atoms (not bound together) in the solid state should be the sum of their van der Waal’s radii. F F van der Waal's radii F F Ionic Radii – Ionic radii were discussed in Chapter 4 and you should go back and review that now. One further thing is worth mentioning here. Evidence that bonding really exists and is attractive can be seen in ionic radii. For all simple ionic compounds, the ions attain noble gas configurations (e.g. in NaCl the Na+ ion is isoelectronic to neon and the Cl- ion is isoelectronic to argon). For the sodium chloride example just given, van der Waal’s radii would predict (Table 8.1, p. -

Organometrallic Chemistry
CHE 425: ORGANOMETALLIC CHEMISTRY SOURCE: OPEN ACCESS FROM INTERNET; Striver and Atkins Inorganic Chemistry Lecturer: Prof. O. G. Adeyemi ORGANOMETALLIC CHEMISTRY Definitions: Organometallic compounds are compounds that possess one or more metal-carbon bond. The bond must be “ionic or covalent, localized or delocalized between one or more carbon atoms of an organic group or molecule and a transition, lanthanide, actinide, or main group metal atom.” Organometallic chemistry is often described as a bridge between organic and inorganic chemistry. Organometallic compounds are very important in the chemical industry, as a number of them are used as industrial catalysts and as a route to synthesizing drugs that would not have been possible using purely organic synthetic routes. Coordinative unsaturation is a term used to describe a complex that has one or more open coordination sites where another ligand can be accommodated. Coordinative unsaturation is a very important concept in organotrasition metal chemistry. Hapticity of a ligand is the number of atoms that are directly bonded to the metal centre. Hapticity is denoted with a Greek letter η (eta) and the number of bonds a ligand has with a metal centre is indicated as a superscript, thus η1, η2, η3, ηn for hapticity 1, 2, 3, and n respectively. Bridging ligands are normally preceded by μ, with a subscript to indicate the number of metal centres it bridges, e.g. μ2–CO for a CO that bridges two metal centres. Ambidentate ligands are polydentate ligands that can coordinate to the metal centre through one or more atoms. – – – For example CN can coordinate via C or N; SCN via S or N; NO2 via N or N. -
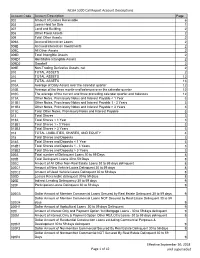
NCUA 5300 Call Report Account Descriptions Page 1 of 42 Effective
NCUA 5300 Call Report Account Descriptions Account Code Account Description Page 002 Amount of Leases Receivable 6 003 Loans Held for Sale 1 007 Land and Building 2 008 Other Fixed Assets 2 009 Total Other Assets 2 009A Accrued Interest on Loans 2 009B Accrued Interest on Investments 2 009C All Other Assets 2 009D Total Intangible Assets 2 009D1 Identifiable Intangible Assets 2 009D2 Goodwill 2 009E Non-Trading Derivative Assets, net 2 010 TOTAL ASSETS 2 010 TOTAL ASSETS 12 010 TOTAL ASSETS 13 010A Average of Daily Assets over the calendar quarter 12 010B Average of the three month-end balances over the calendar quarter 12 010C The average of the current and three preceding calendar quarter-end balances 12 011A Other Notes, Promissory Notes and Interest Payable < 1 Year 3 011B1 Other Notes, Promissory Notes and Interest Payable 1 - 3 Years 3 011B2 Other Notes, Promissory Notes and Interest Payable > 3 Years 3 011C Total Other Notes, Promissory Notes and Interest Payable 3 013 Total Shares 3 013A Total Shares < 1 Year 3 013B1 Total Shares 1 - 3 Years 3 013B2 Total Shares > 3 Years 3 014 TOTAL LIABILITIES, SHARES, AND EQUITY 4 018 Total Shares and Deposits 3 018A Total Shares and Deposits < 1 Year 3 018B1 Total Shares and Deposits 1 - 3 Years 3 018B2 Total Shares and Deposits > 3 Years 3 020A Total number of Delinquent Loans 30 to 59 Days 8 020B Total Delinquent Loans 30 to 59 Days 8 020C Amount of All Other Non-Real Estate Loans 30 to 59 days delinquent 8 020C1 Amount of New Vehicle Loans Delinquent 30 to 59 days 8 020C2 Amount of Used