Battery Sizing Scope
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Study on the Battery State-Of-Charge Estimation Method
ISSN (Print) : 0974-6846 Indian Journal of Science and Technology, Vol 8(26), DOI: 10.17485/ijst/2015/v8i26/81677, October 2015 ISSN (Online) : 0974-5645 Comparative Study on the Battery State-of-Charge Estimation Method Seonwoo Jeon1, Jae-Jung Yun2 and Sungwoo Bae1* 1Department of Electrical Engineering, Yeungnam University, Gyeongsan, Gyeongbuk, Korea; [email protected] 2Department of Electrical and Electronics Engineering, Daegu University, Gyeongsan, Gyeongbuk, Korea Abstract Accurate SOC (State-of-Charge) estimation has been an important part for all applications including energy storage sys- tems. The accurate SOC estimation protects a battery to be deeply discharged and over-charged. Many studies on SOC battery temperature, the type of battery and the external conditions. Because of these reasons, SOC estimation methods differestimation from methodsbattery applications have been developed such as energy for evaluating storage more system, accurate hybrid SOC electrical value. The vehicle battery or electrical SOC can be vehicle. influenced This bypaper the analyzes and compares the strengths and weaknesses of typical estimation methods which have been studied by research- ers. By comparing these advantages and disadvantages of various methods, this paper presents proper estimation methods suitable for energy storage applications. Keywords: Ampere Hour Counting, Electrochemical Impedance Spectroscopy, Open Circuit Voltage, Kalman Filter, SOC (State of Charge) 1. Introduction 2. State-of-Charge Estimation According to the depletion of -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

(Soc) Estimation in Lithium-Ion Battery Electric Vehicles
energies Review Current Trends for State-of-Charge (SoC) Estimation in Lithium-Ion Battery Electric Vehicles Ingvild B. Espedal 1 , Asanthi Jinasena 1 , Odne S. Burheim 1 and Jacob J. Lamb 1,2,* 1 Department of Energy and Process Engineering & ENERSENSE, NTNU, 7491 Trondheim, Norway; [email protected] (I.B.E.); [email protected] (A.J.); [email protected] (O.S.B.) 2 Department of Electronic Systems & ENERSENSE, NTNU, 7491 Trondheim, Norway * Correspondence: [email protected] Abstract: Energy storage systems (ESSs) are critically important for the future of electric vehicles. Despite this, the safety and management of ESSs require improvement. Battery management systems (BMSs) are vital components in ESS systems for Lithium-ion batteries (LIBs). One parameter that is included in the BMS is the state-of-charge (SoC) of the battery. SoC has become an active research area in recent years for battery electric vehicle (BEV) LIBs, yet there are some challenges: the LIB configuration is nonlinear, making it hard to model correctly; it is difficult to assess internal environments of a LIB (and this can be different in laboratory conditions compared to real-world conditions); and these discrepancies can lead to raising the instability of the LIB. Therefore, further advancement is required in order to have higher accuracy in SoC estimation in BEV LIBs. SoC estimation is a key BMS feature, and precise modeling and state estimation will improve stable operation. This review discusses current methods use in BEV LIB SoC modelling and estimation. The review culminates in a brief discussion of challenges in BEV LIB SoC prediction analysis. -
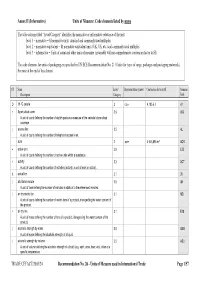
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

Electric Cars: Introduction Glossary of Terms
Electric cars: Introduction Glossary of Terms Alternating current (AC) Type of electric current which periodically switches its direction of flow. Ampere (A) It is the SI unit of electric current, which is equivalent to flow of 1 Coulumb electric charge per second. It is represented by the symbol A. Ampere-hour (Ah) The ampere-hour is a physical unit for measuring electric charge and indicates the charge (often called “capacity” in conversational language) of a battery. One ampere hour is the charge quantity which passes a conductor within 1 hour at a constant current of 1 Ampere. It is denoted by the symbol Ah. Anode An anode is the electrode through which an electric current flows into a polarized electrical device. In an electrochemical device, it is also the electrode at which oxidation reaction occurs. Battery (auxiliary) In an electric drive vehicle, the auxiliary battery provides electricity to start the car before the traction battery is engaged and is also used to power the vehicle accessories. 1 Electric cars: Introduction Glossary of Terms Battery Electric Vehicle (BEV) A type of electric vehicle that solely relies on energy stored in rechargeable battery packs for propulsion. Battery Management System (BMS) It is in the form of a mini-onboard computer to monitor the entire battery system as well as individual batteries. May also be built into the charging system. Battery power converter It is a DC-to-DC power electronic converter that converts the voltage of the traction battery pack to the higher-voltage voltage of the DC-bus used for power exchange with the traction motor. -

Electric Cars: Technology Lecture Notes: Lecture 2.2
Electric cars: Technology Lecture notes: Lecture 2.2 Specific power limitation The ability of a battery to deliver and accept energy at very high rates is limited by the physical processes occurring within the battery cells. When current flows into the battery, the reaction within the cell must occur at a corresponding rate1. This means that the dynamics of the reaction at the electrode surface and the transport of ions (kinetic properties) must occur at the same rate as the supplied current. Because of the high currents associated with high power, the reaction rate is unable to match the rate at which current is being supplied. As a result, the capacity of the battery is reduced and joule heating occurs within the cell. Specific energy limitation The restricted energy content of batteries is one of the major drawbacks limiting the successful implementation of EV technology. Considering the specific energy of gasoline is 9.2kWhkg-1 corresponding to more than 3kWhkg-1 useful specific energy2, limitations of the battery powered EV become apparent. Two emerging battery technologies addressing the specific energy limitations are lithium air (Li- air) and lithium flour (Li-flour). 1 Electric cars: Technology Lecture notes: Lecture 2.2 Summary of equations 1. Nominal energy capacity (Enom, in Wh or kWh): It is the maximum amount of electrical energy that can be extracted from a fully charged battery state to the empty state. 2. Nominal voltage (Vnom, in V) : It is rated voltage of the battery when it is fully charged. When a battery is discharged or is loaded, the voltage reduces gradually to a lower value, Vbatt . -

ECPC404 Intelligent Ampere Hour Meter
ECPC404 Intelligent Ampere Hour Meter OPERATION MANUAL INTRODUCTION This Operations Manual describes the use and configuration of the ECPC404 Intelligent AH meter. The ECPC404 allows reasonably accurate measurement of large values of DC voltage, current and the integration of ampere hours across time. It is useful for solar power installations but particularly for electric vehicles where current levels in excess of 1000 amperes and voltages as high as 500v are required. In measuring Ampere Hours or AH, the ECPC404 can provide a numeric indication of the state of charge (SOC ) of Lithium Ion batteries that can be difficult to determine using voltage measurem ent. The ECPC404 provides four basic measurement functions: 1. Ampere Hours – current flow integrated over time. 2. Time – hours and minutes of elapsed time 3. DC Amperes – instantaneous current up to 9999 amps 4. DC Volts – up to 500 VD C Additionally, the ECPC404 can perform any number of control functions via two control relays, J1 and J2, which can be activated by any of the four basic measurement functions, and EACH relay can be activated/deactivated at TWO separate values. In this way, you can not only observe your battery state of charge, voltage, etc. but you can take action automatically based on that State of Charge level. PACKING LIST 1. ECPC404 panel meter 2. 75 millivolt current shunt 3. This manual SPECIFICATIONS The ECPC404 Panel Meter offers the following features/specifications: Current Sensor Inputs: 5A, 1A, 75mv DC Voltage Inputs: -100/500vdc, -20/100vdc Current Measurement: 0-9999 amperes with external shunt. 0.5%FS+3D Voltage Measurement: -100 to +500 vdc. -

Glossary: Technical Battery Terms
GLOSSARY: TECHNICAL BATTERY TERMS A metal alloy (e.g., LaNi5) capable of undergoing a reversible AB5 hydrogen absorption/desorption reaction as the battery is charged and discharged, respectively. The taking up or retention of one material by another by chemical Absorption or molecular action. Alloy A mixture of several other metals or a metal and a non-metal. Ambient Humidity The average humidity of the surroundings. Ambient Temperature The average temperature of the surroundings. The quantity of electricity measured in ampere-hours (Ah) which Ampere-Hour Capacity may be delivered by a cell or battery under specified conditions. The electrode in an electrochemical cell where oxidation takes Anode place. During discharge, the negative electrode of the cell is the anode. During charge, the positive electrode is the anode. Two or more electrochemical cells electrically interconnected in an appropriate series/parallel arrangement to provide the required Battery or Pack operating voltage and current levels. Under common usage, the term "battery" is often also applied to a single cell. A cylindrical cell design utilizing an internal cylindrical electrode, Bobbin and an external electrode arranged as a sleeve inside the cell container. Discharge or charge current, in amperes, expressed in multiples of the rated capacity. For example, C/10 discharge current for a C-Rate (also see Hourly battery rated at 1.5 Ah is: Rate) 1.5 Ah/10 = 150 mA (A cell's capacity is not the same at all discharge rates and usually increases with decreasing rate.) The total number of ampere-hours or watt-hours that can be Capacity withdrawn from a fully charged cell or battery under specified conditions of discharge. -

Electro- Chemical Power Sources
CHAPTER9 Learning Objectives ➣➣➣ Faraday’s Laws of electrolysis ➣➣➣ Value of Back e.m.f. ELECTRO- ➣➣➣ Primary and Secondary Batteries ➣➣➣ Classification of Lead Storage CHEMICAL Batteries ➣➣➣ Formation of Plates of Lead- acid Cells POWER ➣➣➣ Plante Process ➣➣➣ Faure Process ➣➣➣ Positive Pasted Plates ➣➣➣ Negative Pasted Plates SOURCES ➣➣➣ Comparison : Plante and Faure Plates ➣➣➣ Internal Resistance and Capacity of a Cell ➣➣➣ Electrical Characteristics of the Lead-acid Cell ➣➣➣ Battery Ratings ➣➣➣ Indications of a Fully-Charged Cell ➣➣➣ Voltage Regulators ➣➣➣ End-cell Control System ➣➣➣ Charging Systems ➣➣➣ Constant-current System- Constant-voltage System ➣➣➣ Trickle Charging ➣➣➣ Sulphation-Causes and Cure ➣➣➣ Mains operated Battery Chargers ➣➣➣ Car Battery Charger ➣➣➣ Automobile Battery Charger ➣➣➣ Static Uninterruptable Power Systems ➣➣➣ Alkaline Batteries ➣➣➣ Nickel-iron or Edison Batteries ➣➣➣ Nickel-Cadmium Batteries © Electrochemical cells convert ➣➣➣ Silver-zinc Batteries chemical energy into electrical ➣➣➣ High Temperature Batteries energy ➣➣➣ Secondary Hybrid Cells ➣➣➣ Fuel Cells 340 Electrical Technology 9.1. Faraday’s Laws of Electrolysis From his experiments, Faraday deduced two fundamental laws which govern the phenomenon of electrolysis. These are : (i) First Law. The mass of ions liberated at an electrode is directly proportional to the quantity of electricity i.e. charge which passes through the electrolyte. (ii) Second Law. The masses of ions of different substances liberated by the same quantity of electricity are proportional to their chemical equivalent weights. Explanation of the First Law Michael Faraday If m = mass of the ions liberated, Q = quantity of electricity = I × t where I is the current and t is the time, then according to the first law m α Q or m = Z Q or m = Z It where Z is a constant and is known as the electrochemical equivalent (E.C.E.) of the substance. -

Battery Capacity Analyzer with Record Storage Model 603B
Data Sheet Battery Capacity Analyzer with record storage Model 603B Features & benefits The 603B handheld battery capacity analyzer tests 6 and 12 volt sealed lead acid batteries with n Test 6 and 12 volt batteries up to 100 Ah capacities up to 100 ampere hour (Ah). Test n Test both open and loaded battery voltage results include voltage, state of charge, and n Powered by the battery under test (no need to internal resistance. This analyzer also features a replace battery) built-in USB port and internal memory to store n Fast test cycle time for quick sorting of battery information, test configuration, and measurement results for up to 50 batteries. batteries n Measurements are stored to internal memory n Pre-load up to 50 test configurations from the computer via USB Application n Test configurations include site, panel, battery Ideal choice for testing sealed lead acid batteries commonly used in intrusion information and more detection, fire alarm, security camera, access n Add or edit records using the key pad or control, industrial control and other battery remote computer backup systems. n Real-time clock for date and time stamp of measurement records n Export record data to a CSV file for Fast results further analysis Getting battery measurement results fast is critical n User configurable Pass/Fail criteria in the field. Immediately upon connection, the n Built-in charger circuit test 603B measures and displays open battery voltage. Battery voltage under load, state of charge and internal resistance are determined within seconds, by simply entering the battery ampere hour (Ah) n Twist-lock test leads can be changed in seconds rating. -
The SI Metric Systeld of Units and SPE METRIC STANDARD
The SI Metric SystelD of Units and SPE METRIC STANDARD Society of Petroleum Engineers The SI Metric System of Units and SPE METRIC STANDARD Society of Petroleum Engineers Adopted for use as a voluntary standard by the SPE Board of Directors, June 1982. Contents Preface . ..... .... ......,. ............. .. .... ........ ... .. ... 2 Part 1: SI - The International System of Units . .. .. .. .. .. .. .. .. ... 2 Introduction. .. .. .. .. .. .. .. .. .. .. .. .. 2 SI Units and Unit Symbols. .. .. .. .. .. .. .. .. .. .. .. 2 Application of the Metric System. .. .. .. .. .. .. .. .. .. .. .. .. 3 Rules for Conversion and Rounding. .. .. .. .. .. .. .. .. .. .. .. .. 5 Special Terms and Quantities Involving Mass and Amount of Substance. .. 7 Mental Guides for Using Metric Units. .. .. .. .. .. .. .. .. .. .. .. .. .. 8 Appendix A (Terminology).. .. .. .. .. .. .. .. .. .. .. .. .. 8 Appendix B (SI Units). .. .. .. .. .. .. .. .. .. .. .. .. 9 Appendix C (Style Guide for Metric Usage) ............ ...... ..... .......... 11 Appendix D (General Conversion Factors) ................... ... ........ .. 14 Appendix E (Tables 1.8 and 1.9) ......................................... 20 Part 2: Discussion of Metric Unit Standards. .. .. .. .. .. .. .. .. 21 Introduction.. .. .. .. .. .. .. .. .. .. .. .. 21 Review of Selected Units. .. .. .. .. .. .. .. .. .. .. 22 Unit Standards Under Discussion ......................................... 24 Notes for Table 2.2 .................................................... 25 Notes for Table 2.3 ................................................... -

2.3 WORK in ELECTRICAL SYSTEMS 107 Figure 2.14 a Battery Moves Charge Off One Plate and Onto the Other
Objectives • Explain the relationship between work done in electrical systems, charge moved, and the potential difference. • Calculate the amount of electrical charge in coulombs moving past a point in a circuit. • Define the units of electric current. • Solve electrical work problems, given voltage and charge information. • Identify the effects of work done in electrical systems. • Explain how efficiency relates to input work and output work in an electrical system. A force does work on an object when it moves the object. In an electrical system, forces are applied to charged objects by electric fields. Electric fields are created by other charges. You learned in Section 1.3 that a collection of charges creates an electric To find out more about work in electrical systems, field. When there is an electric potential (or voltage) difference between two follow the links at points within the field, a force is exerted on a charge placed at one of the www.learningincontext.com. points. If you connect the two points with a conductor (a piece of metal or a wire), the force causes charge to move from one point toward the other through the conductor. For this reason, we say that potential difference is the 106 CHAPTER 2 WORK prime mover in electrical systems. Just as the presence of a pressure difference can result in a fluid moving and doing work in a fluid system, the presence of a potential difference can result in charge moving and doing work in an electrical system. Examples of electrical systems are shown in Figure 2.13. Notice that, in each example, you can’t see the electrical work being done.