Activity-Dependent Aberrations in Gene Expression and Alternative
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcriptomic Profiling of Equine and Viral Genes in Peripheral Blood
pathogens Article Transcriptomic Profiling of Equine and Viral Genes in Peripheral Blood Mononuclear Cells in Horses during Equine Herpesvirus 1 Infection Lila M. Zarski 1, Patty Sue D. Weber 2, Yao Lee 1 and Gisela Soboll Hussey 1,* 1 Department of Pathobiology and Diagnostic Investigation, Michigan State University, East Lansing, MI 48824, USA; [email protected] (L.M.Z.); [email protected] (Y.L.) 2 Department of Large Animal Clinical Sciences, Michigan State University, East Lansing, MI 48824, USA; [email protected] * Correspondence: [email protected] Abstract: Equine herpesvirus 1 (EHV-1) affects horses worldwide and causes respiratory dis- ease, abortions, and equine herpesvirus myeloencephalopathy (EHM). Following infection, a cell- associated viremia is established in the peripheral blood mononuclear cells (PBMCs). This viremia is essential for transport of EHV-1 to secondary infection sites where subsequent immunopathol- ogy results in diseases such as abortion or EHM. Because of the central role of PBMCs in EHV-1 pathogenesis, our goal was to establish a gene expression analysis of host and equine herpesvirus genes during EHV-1 viremia using RNA sequencing. When comparing transcriptomes of PBMCs during peak viremia to those prior to EHV-1 infection, we found 51 differentially expressed equine genes (48 upregulated and 3 downregulated). After gene ontology analysis, processes such as the interferon defense response, response to chemokines, the complement protein activation cascade, cell adhesion, and coagulation were overrepresented during viremia. Additionally, transcripts for EHV-1, EHV-2, and EHV-5 were identified in pre- and post-EHV-1-infection samples. Looking at Citation: Zarski, L.M.; Weber, P.S.D.; micro RNAs (miRNAs), 278 known equine miRNAs and 855 potentially novel equine miRNAs were Lee, Y.; Soboll Hussey, G. -

Autism Multiplex Family with 16P11.2P12.2 Microduplication Syndrome in Monozygotic Twins and Distal 16P11.2 Deletion in Their Brother
European Journal of Human Genetics (2012) 20, 540–546 & 2012 Macmillan Publishers Limited All rights reserved 1018-4813/12 www.nature.com/ejhg ARTICLE Autism multiplex family with 16p11.2p12.2 microduplication syndrome in monozygotic twins and distal 16p11.2 deletion in their brother Anne-Claude Tabet1,2,3,4, Marion Pilorge2,3,4, Richard Delorme5,6,Fre´de´rique Amsellem5,6, Jean-Marc Pinard7, Marion Leboyer6,8,9, Alain Verloes10, Brigitte Benzacken1,11,12 and Catalina Betancur*,2,3,4 The pericentromeric region of chromosome 16p is rich in segmental duplications that predispose to rearrangements through non-allelic homologous recombination. Several recurrent copy number variations have been described recently in chromosome 16p. 16p11.2 rearrangements (29.5–30.1 Mb) are associated with autism, intellectual disability (ID) and other neurodevelopmental disorders. Another recognizable but less common microdeletion syndrome in 16p11.2p12.2 (21.4 to 28.5–30.1 Mb) has been described in six individuals with ID, whereas apparently reciprocal duplications, studied by standard cytogenetic and fluorescence in situ hybridization techniques, have been reported in three patients with autism spectrum disorders. Here, we report a multiplex family with three boys affected with autism, including two monozygotic twins carrying a de novo 16p11.2p12.2 duplication of 8.95 Mb (21.28–30.23 Mb) characterized by single-nucleotide polymorphism array, encompassing both the 16p11.2 and 16p11.2p12.2 regions. The twins exhibited autism, severe ID, and dysmorphic features, including a triangular face, deep-set eyes, large and prominent nasal bridge, and tall, slender build. The eldest brother presented with autism, mild ID, early-onset obesity and normal craniofacial features, and carried a smaller, overlapping 16p11.2 microdeletion of 847 kb (28.40–29.25 Mb), inherited from his apparently healthy father. -

Accepted By:- Signature Redacted Department of Biology Graduate Co-Chair: Michael T
EXPLORING THE FUNCTIONAL CONSERVATION OF MUSCLEBLIND (MBL) PROTEINS by MASSACH USETTS INSTITUTE Julia C. Oddo d o ECHNOLOGY B.S. Molecular, Cellular, and Developmental Biology, SE 1 7 2015 Minor Biochemistry 2011 LIB RARIES SUBMITTED TO THE DEPARTMENT OF BIOLOGY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTERS OF SCIENCE IN BIOLOGY AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY SEPTEMBER 2015 C 2015 Massachusetts Institute of Technology. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature redacted Signature of Auth or:_ Department of Biology May 22, 2015 Certified by: Signature redacte Thesis Supervisor: Chris B. Burge Professor of Biology and Biological Engineering Director Program CSB Certified by: Signature redactec Thesis Co-Supervisor: Eric T. Wang Research Fellow Accepted by:- Signature redacted Department of Biology Graduate Co-Chair: Michael T. Hemann Associate Professor of Biology 1 EXPLORING THE FUNCTIONAL CONSERVATION OF MUSCLEBLIND (MBL) PROTEINS by Julia C. Oddo Submitted to the Department of Biology on September 8th, 2015 in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biology ABSTRACT Muscleblind (Mbl) is an evolutionarily conserved family of proteins involved in many aspects of RNA metabolism, including alternative splicing. Disruption of Muscleblind in several animals lends to a variety of defects and disease, including the multi-systemic disorder Myotonic Dystrophy (DM). Though much is known about the involvement of Muscleblind in DM, there is much basic knowledge of the protein's function to be discovered. -

Mapping of Diabetes Susceptibility Loci in a Domestic Cat Breed with an Unusually High Incidence of Diabetes Mellitus
G C A T T A C G G C A T genes Article Mapping of Diabetes Susceptibility Loci in a Domestic Cat Breed with an Unusually High Incidence of Diabetes Mellitus 1,2, 3, 4 5 Lois Balmer y , Caroline Ann O’Leary y, Marilyn Menotti-Raymond , Victor David , Stephen O’Brien 6,7, Belinda Penglis 8, Sher Hendrickson 9, Mia Reeves-Johnson 3, 3 10 3 3,11, Susan Gottlieb , Linda Fleeman , Dianne Vankan , Jacquie Rand z and 1, , Grant Morahan * z 1 Centre for Diabetes Research, Harry Perkins Institute for Medical Research, University of Western Australia, Nedlands 6009, Australia; [email protected] 2 School of Medical and Health Sciences, Edith Cowan University, Joondalup, Perth 6027, Australia 3 School of Veterinary Science, the University of Queensland, Gottan 4343, Australia; [email protected] (C.A.O.); [email protected] (M.R.-J.); [email protected] (S.G.); [email protected] (D.V.); [email protected] (J.R.) 4 Laboratory of Genomic Diversity, Center for Cancer Research (FNLCR), Frederick, MD 21702, USA; [email protected] 5 Laboratory of Basic Research, Center for Cancer Research (FNLCR), National Cancer Institute, Frederick, MD 21702, USA; [email protected] 6 Laboratory of Genomics Diversity, Center for Computer Technologies, ITMO University, 197101 St. Petersburg, Russia; [email protected] 7 Guy Harvey Oceanographic Center, Halmos College of Arts and Sciences, Nova Southeastern University, Ft Lauderdale, FL 33004, USA 8 IDEXX Laboratories, East Brisbane 4169, Australia; [email protected] 9 Department of Biology, Shepherd University, Shepherdstown, WV 25443, USA; [email protected] 10 Animal Diabetes Australia, Melbourne 3155, Australia; l.fl[email protected] 11 American College of Veterinary Internal Medicine, University of Zurich, 8006 Zurich, Switzerland * Correspondence: [email protected] These authors contributed equally to this work. -

Supplementary Materials
Supplementary materials Supplementary Table S1: MGNC compound library Ingredien Molecule Caco- Mol ID MW AlogP OB (%) BBB DL FASA- HL t Name Name 2 shengdi MOL012254 campesterol 400.8 7.63 37.58 1.34 0.98 0.7 0.21 20.2 shengdi MOL000519 coniferin 314.4 3.16 31.11 0.42 -0.2 0.3 0.27 74.6 beta- shengdi MOL000359 414.8 8.08 36.91 1.32 0.99 0.8 0.23 20.2 sitosterol pachymic shengdi MOL000289 528.9 6.54 33.63 0.1 -0.6 0.8 0 9.27 acid Poricoic acid shengdi MOL000291 484.7 5.64 30.52 -0.08 -0.9 0.8 0 8.67 B Chrysanthem shengdi MOL004492 585 8.24 38.72 0.51 -1 0.6 0.3 17.5 axanthin 20- shengdi MOL011455 Hexadecano 418.6 1.91 32.7 -0.24 -0.4 0.7 0.29 104 ylingenol huanglian MOL001454 berberine 336.4 3.45 36.86 1.24 0.57 0.8 0.19 6.57 huanglian MOL013352 Obacunone 454.6 2.68 43.29 0.01 -0.4 0.8 0.31 -13 huanglian MOL002894 berberrubine 322.4 3.2 35.74 1.07 0.17 0.7 0.24 6.46 huanglian MOL002897 epiberberine 336.4 3.45 43.09 1.17 0.4 0.8 0.19 6.1 huanglian MOL002903 (R)-Canadine 339.4 3.4 55.37 1.04 0.57 0.8 0.2 6.41 huanglian MOL002904 Berlambine 351.4 2.49 36.68 0.97 0.17 0.8 0.28 7.33 Corchorosid huanglian MOL002907 404.6 1.34 105 -0.91 -1.3 0.8 0.29 6.68 e A_qt Magnogrand huanglian MOL000622 266.4 1.18 63.71 0.02 -0.2 0.2 0.3 3.17 iolide huanglian MOL000762 Palmidin A 510.5 4.52 35.36 -0.38 -1.5 0.7 0.39 33.2 huanglian MOL000785 palmatine 352.4 3.65 64.6 1.33 0.37 0.7 0.13 2.25 huanglian MOL000098 quercetin 302.3 1.5 46.43 0.05 -0.8 0.3 0.38 14.4 huanglian MOL001458 coptisine 320.3 3.25 30.67 1.21 0.32 0.9 0.26 9.33 huanglian MOL002668 Worenine -

Nuclear PTEN Safeguards Pre-Mrna Splicing to Link Golgi Apparatus for Its Tumor Suppressive Role
ARTICLE DOI: 10.1038/s41467-018-04760-1 OPEN Nuclear PTEN safeguards pre-mRNA splicing to link Golgi apparatus for its tumor suppressive role Shao-Ming Shen1, Yan Ji2, Cheng Zhang1, Shuang-Shu Dong2, Shuo Yang1, Zhong Xiong1, Meng-Kai Ge1, Yun Yu1, Li Xia1, Meng Guo1, Jin-Ke Cheng3, Jun-Ling Liu1,3, Jian-Xiu Yu1,3 & Guo-Qiang Chen1 Dysregulation of pre-mRNA alternative splicing (AS) is closely associated with cancers. However, the relationships between the AS and classic oncogenes/tumor suppressors are 1234567890():,; largely unknown. Here we show that the deletion of tumor suppressor PTEN alters pre-mRNA splicing in a phosphatase-independent manner, and identify 262 PTEN-regulated AS events in 293T cells by RNA sequencing, which are associated with significant worse outcome of cancer patients. Based on these findings, we report that nuclear PTEN interacts with the splicing machinery, spliceosome, to regulate its assembly and pre-mRNA splicing. We also identify a new exon 2b in GOLGA2 transcript and the exon exclusion contributes to PTEN knockdown-induced tumorigenesis by promoting dramatic Golgi extension and secretion, and PTEN depletion significantly sensitizes cancer cells to secretion inhibitors brefeldin A and golgicide A. Our results suggest that Golgi secretion inhibitors alone or in combination with PI3K/Akt kinase inhibitors may be therapeutically useful for PTEN-deficient cancers. 1 Department of Pathophysiology, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine (SJTU-SM), Shanghai 200025, China. 2 Institute of Health Sciences, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences and SJTU-SM, Shanghai 200025, China. -

Dissertation
Regulation of gene silencing: From microRNA biogenesis to post-translational modifications of TNRC6 complexes DISSERTATION zur Erlangung des DOKTORGRADES DER NATURWISSENSCHAFTEN (Dr. rer. nat.) der Fakultät Biologie und Vorklinische Medizin der Universität Regensburg vorgelegt von Johannes Danner aus Eggenfelden im Jahr 2017 Das Promotionsgesuch wurde eingereicht am: 12.09.2017 Die Arbeit wurde angeleitet von: Prof. Dr. Gunter Meister Johannes Danner Summary ‘From microRNA biogenesis to post-translational modifications of TNRC6 complexes’ summarizes the two main projects, beginning with the influence of specific RNA binding proteins on miRNA biogenesis processes. The fate of the mature miRNA is determined by the incorporation into Argonaute proteins followed by a complex formation with TNRC6 proteins as core molecules of gene silencing complexes. miRNAs are transcribed as stem-loop structured primary transcripts (pri-miRNA) by Pol II. The further nuclear processing is carried out by the microprocessor complex containing the RNase III enzyme Drosha, which cleaves the pri-miRNA to precursor-miRNA (pre-miRNA). After Exportin-5 mediated transport of the pre-miRNA to the cytoplasm, the RNase III enzyme Dicer cleaves off the terminal loop resulting in a 21-24 nt long double-stranded RNA. One of the strands is incorporated in the RNA-induced silencing complex (RISC), where it directly interacts with a member of the Argonaute protein family. The miRNA guides the mature RISC complex to partially complementary target sites on mRNAs leading to gene silencing. During this process TNRC6 proteins interact with Argonaute and recruit additional factors to mediate translational repression and target mRNA destabilization through deadenylation and decapping leading to mRNA decay. -

An Ancient Germ Cell-Specific RNA-Binding Protein Protects
RESEARCH ARTICLE An ancient germ cell-specific RNA-binding protein protects the germline from cryptic splice site poisoning Ingrid Ehrmann1, James H Crichton2, Matthew R Gazzara3,4, Katherine James5, Yilei Liu1,6, Sushma Nagaraja Grellscheid1,7, TomazˇCurk8, Dirk de Rooij9,10, Jannetta S Steyn11, Simon Cockell11, Ian R Adams2, Yoseph Barash3,12*, David J Elliott1* 1Institute of Genetic Medicine, Newcastle University, Newcastle, United Kingdom; 2MRC Human Genetics Unit, MRC Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, United Kingdom; 3Department of Genetics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, United States; 4Department of Biochemistry and Biophysics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, United States; 5Life Sciences, Natural History Museum, London, United Kingdom; 6Department of Plant and Microbial Biology, University of Zu¨ rich, Zu¨ rich, Switzerland; 7School of Biological and Biomedical Sciences, University of Durham, Durham, United Kingdom; 8Laboratory of Bioinformatics, Faculty of Computer and Information Sciences, University of Ljubljana, Ljubljana, Slovenia; 9Reproductive Biology Group, Division of Developmental Biology, Department of Biology, Faculty of Science, Utrecht University, Utrecht, The Netherlands; 10Center for Reproductive Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; 11Bioinformatics Support Unit, Faculty of Medical Sciences, Newcastle University, Newcastle, United Kingdom; 12Department of Computer and Information Science, University of Pennsylvania, Philadelphia, United States *For correspondence: [email protected] (YB); [email protected] (DJE) Competing interests: The Abstract Male germ cells of all placental mammals express an ancient nuclear RNA binding authors declare that no protein of unknown function called RBMXL2. Here we find that deletion of the retrogene encoding competing interests exist. -

Review Article Expression of Tra2 in Cancer Cells As a Potential Contributory Factor to Neoplasia and Metastasis
Hindawi Publishing Corporation International Journal of Cell Biology Volume 2013, Article ID 843781, 9 pages http://dx.doi.org/10.1155/2013/843781 Review Article Expression of Tra2 in Cancer Cells as a Potential Contributory Factor to Neoplasia and Metastasis Andrew Best,1 Caroline Dagliesh,1 Ingrid Ehrmann,1 Mahsa Kheirollahi-Kouhestani,1 Alison Tyson-Capper,2 and David J. Elliott1 1 Institute of Genetic Medicine, Newcastle University, Central Parkway, Newcastle upon Tyne NE1 3BZ, UK 2 Institute of Cellular Medicine, Newcastle University, Framlington Place, Newcastle upon Tyne NE2 4HH, UK Correspondence should be addressed to David J. Elliott; [email protected] Received 2 May 2013; Accepted 9 June 2013 Academic Editor: Claudia Ghigna Copyright © 2013 Andrew Best et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The splicing regulator proteins SRSF1 (also known as ASF/SF2) and SRSF3 (also known as SRP20) belong to the SR family of proteins and can be upregulated in cancer. The SRSF1 gene itself is amplified in some cancer cells, and cancer-associated changes in the expression of MYC also increase SRSF1 gene expression. Increased concentrations of SRSF1 protein promote prooncogenic splicing patterns of a number of key regulators of cell growth. Here, we review the evidence that upregulation of the SR-related Tra2 protein might have a similar role in cancer cells. The TRA2B gene encoding Tra2 is amplified in particular tumours including those of the lung, ovary, cervix, stomach, head, and neck. -
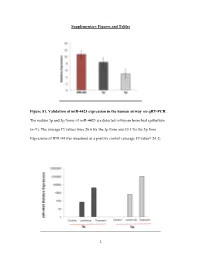
1 Supplementary Figures and Tables Figure S1. Validation
Supplementary Figures and Tables Figure S1. Validation of miR-4423 expression in the human airway via qRT-PCR. The mature 3p and 5p forms of miR-4423 are detected in human bronchial epithelium (n=9). The average Ct values were 26.6 for the 3p form and 30.1 for the 5p form. Expression of RNU44 was measured as a positive control (average Ct value= 24.3). 1 Figure S2. MiR-4423 overexpression. A) Stable and transient overexpression of a plasmid containing the miR-4423 precursor plus 200 bps of flanking region, results in the production of processed 3p and 5p forms. An empty vector was used as a control. Figure S3. Validation of miR-4423 biogenesis. A) Transfection with a siRNA targeting Dicer decreases Dicer mRNA levels in H1299 cells stably overexpressing miR-4423 (P=0.0001)(n=3). B) Levels of processed miR-4423-5p, miR-4423-3p, miR-10b, miR-21 and miR-26 are significantly decreased in Dicer knocked-down cells compared to control (P= 0.0014, P= 0.0013, P=0.04, P=0.01; P=0.019, respectively). Error bars indicate standard error and P values were determined using Student’s t test. 2 Figure S4. RNA-Seq profile of the miR-4423 locus using reads that associated with the AGO complex. Using a small RNA sequencing dataset from Hafner et al (1), one read that associated with TNRC6C and two reads that associated with AGO3 aligned uniquely to the 5p form while one read that associated with TNRC6A aligned uniquely to the 3p form. -

Identification and Characterisation of RNA Targets of the RNA Binding Proteins Tra2α and Tra2β Andrew Best
Identification and characterisation of RNA targets of the RNA binding proteins Tra2α and Tra2β Andrew Best Institute of Genetic Medicine A thesis submitted for the degree of Doctor of Philosophy September 2014 Declaration Declaration I, Andrew Best, declare that no portion of the work compiled in this thesis has been submitted in support of another degree or qualification at this or any other University or Institute of Learning. This thesis includes nothing which is the work of others, nor the outcomes of work done in collaboration, except where otherwise stated. ……………………………….. Andrew Best I Acknowledgements Acknowledgements First and foremost, I would like to extend my thanks to a fantastic supervisor - Professor David Elliott – for his guidance, patience and support. David has been a kind and enthusiastic mentor. I would also like to thank my second supervisor – Dr Alison Tyson-Capper – for her constant encouragement and support throughout this project. I would like to thank all members of the Elliott lab, both past and present. I would particularly like to thank Mrs Caroline Dalgliesh (Institute of Genetic Medicine, Newcastle University) for her valuable advice and for the countless ‘quick questions’ she has kindly answered over the years. Thanks to Dr Sushma Grellscheid (School of Biological and Biomedical Sciences, Durham University) for sharing her valuable expertise when I first started working in the lab. I would also like to thank Dr Jennifer Munkley, Dr Elaine Hong and Dr Ingrid Ehrmann for their help and advice. You have all made my PhD an enjoyable and rewarding experience. I extend a special thank you to my good friends and fellow PhD students Ms Marina Danilenko, Mr Morten Ritso and Dr Mahsa Kheirollahi-Kouhestani. -

A High-Throughput Approach to Uncover Novel Roles of APOBEC2, a Functional Orphan of the AID/APOBEC Family
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2018 A High-Throughput Approach to Uncover Novel Roles of APOBEC2, a Functional Orphan of the AID/APOBEC Family Linda Molla Follow this and additional works at: https://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons A HIGH-THROUGHPUT APPROACH TO UNCOVER NOVEL ROLES OF APOBEC2, A FUNCTIONAL ORPHAN OF THE AID/APOBEC FAMILY A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy by Linda Molla June 2018 © Copyright by Linda Molla 2018 A HIGH-THROUGHPUT APPROACH TO UNCOVER NOVEL ROLES OF APOBEC2, A FUNCTIONAL ORPHAN OF THE AID/APOBEC FAMILY Linda Molla, Ph.D. The Rockefeller University 2018 APOBEC2 is a member of the AID/APOBEC cytidine deaminase family of proteins. Unlike most of AID/APOBEC, however, APOBEC2’s function remains elusive. Previous research has implicated APOBEC2 in diverse organisms and cellular processes such as muscle biology (in Mus musculus), regeneration (in Danio rerio), and development (in Xenopus laevis). APOBEC2 has also been implicated in cancer. However the enzymatic activity, substrate or physiological target(s) of APOBEC2 are unknown. For this thesis, I have combined Next Generation Sequencing (NGS) techniques with state-of-the-art molecular biology to determine the physiological targets of APOBEC2. Using a cell culture muscle differentiation system, and RNA sequencing (RNA-Seq) by polyA capture, I demonstrated that unlike the AID/APOBEC family member APOBEC1, APOBEC2 is not an RNA editor. Using the same system combined with enhanced Reduced Representation Bisulfite Sequencing (eRRBS) analyses I showed that, unlike the AID/APOBEC family member AID, APOBEC2 does not act as a 5-methyl-C deaminase.