Elattostachys Microcarpa Click on Images to Enlarge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No. 110 MARCH 2002 Price: $5.00
No. 110 MARCH 2002 Price: $5.00 AUSTRALIAN SYSTEMATIC BOTANY SOCIETY INCORPORATED Office Bearers President Vice President Barry Conn W.R.(Bill) Barker Royal Botanic Gardens Sydney Plant Biodiversity Centre Mrs Macquaries Road Hackney Road Sydney NSW 2000 Hackney SA 5069 tel: (02) 9231 8131 tel: (08) 82229303 email: [email protected] email: [email protected] Secretary Treasurer Brendan Lepschi Anthony Whalen Centre for Plant Biodiversity Research Centre for Plant Biodiversity Research Australian National Herbarium Australian National Herbarium GPO Box 1600, Canberra GPO Box 1600, Canberra ACT 2601 ACT 2601 tel: (02) 6246 5167 tel: (02) 6246 5175 email: [email protected] email: [email protected] Councillor Councillor Andrew Rozefelds R.O.(Bob) Makinson Tasmanian Herbarium Royal Botanic Gardens Sydney GPO Box 252-40 Mrs Macquaries Road Hobart, Tasmania 7001 Sydney NSW 2000 tel.: (03) 6226 2635 tel: (02) 9231 8111 email: [email protected] email: [email protected] Public Officer Annette Wilson Australian Biological Resources Study Environment Australia GPO Box 787 CANBERRA ACT 2601 tel: (02) 6250 9417 email: [email protected] Affiliate Society Papua New Guinea Botanical Society ASBS Web site http://www.anbg.gov.au/asbs Publication dates of previous issue Austral.Syst.Bot.Soc.Nsltr 109 (December 2001 issue) Hardcopy: 15th Jan 2002; ASBS Web site: 15th Jan 2002 Australian Systematic Botany Society Newsletter 110 (March 2002) ASBS Inc. Business Council elections with at the Annual General Meeting, which is more than four months after lodgement as A slip for nominations to the next Council is required by the Rules. -

9 Costion Plant Endemism 133-166 PROOFS
Micronesica 41(1): 131–164, 2009 Plant Endemism, Rarity, and Threat in Palau, Micronesia: A Geographical Checklist and Preliminary Red List Assessment 1 CRAIG M. COSTION Department of Ecology and Evolutionary Biology, School of Earth and Environmental Sciences, University of Adelaide, Adelaide SA 5001 [email protected] ANN HILLMANN KITALONG The Environment, Inc., P.O. Box 1696, Koror, Palau 96940 TARITA HOLM Palau Conservation Society/PALARIS, P.O. Box 1811, Koror, Palau, 96940 Abstract—An official checklist of the endemic plant species of Palau has been long awaited, and is presented here for the first time. For each species a substrate limitation, growth form, and relative abundance is listed. In addition an IUCN red list assessment was conducted using all available data. For over half of the endemic species there is insufficient data to provide a red listing status however an expected minimum number of threatened plants out of the total is inferred. Approximately 15% of Palau’s endemic plants are believed to be only known from the type collection and many more only known from a few collections. These taxa however may now be prioritized and targeted for future inventory and research. The taxonomic robustness of several of these taxa is questionable and it is expected that more endemic species will be lost to synonymy in the future. Previous estimations have significantly over-estimated the rate of plant endemism in Palau (e.g., 194). Here, 130 plants are recognized for Palau, making its level of plant endem- ism comparable to some of its neighboring Micronesian islands to the east, notably Guam and Pohnpei. -

Accepted Manuscript
Accepted Manuscript Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae) Sven Buerki, Félix Forest, Pedro Acevedo-Rodríguez, Martin W. Callmander, Johan A.A. Nylander, Mark Harrington, Isabel Sanmartín, Philippe Küpfer, Nadir Alvarez PII: S1055-7903(09)00017-7 DOI: 10.1016/j.ympev.2009.01.012 Reference: YMPEV 3130 To appear in: Molecular Phylogenetics and Evolution Received Date: 21 May 2008 Revised Date: 27 November 2008 Accepted Date: 23 January 2009 Please cite this article as: Buerki, S., Forest, F., Acevedo-Rodríguez, P., Callmander, M.W., Nylander, J.A.A., Harrington, M., Sanmartín, I., Küpfer, P., Alvarez, N., Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae), Molecular Phylogenetics and Evolution (2009), doi: 10.1016/j.ympev.2009.01.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT Buerki et al. 1 1 Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal 2 levels in the soapberry family (Sapindaceae) 3 4 Sven Buerki a,*, Félix Forest b, Pedro Acevedo-Rodríguez c, Martin W. Callmander d,e, 5 Johan A. -

Plastid and Nuclear DNA Markers.Pdf
Molecular Phylogenetics and Evolution 51 (2009) 238–258 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae) Sven Buerki a,*, Félix Forest b, Pedro Acevedo-Rodríguez c, Martin W. Callmander d,e, Johan A.A. Nylander f, Mark Harrington g, Isabel Sanmartín h, Philippe Küpfer a, Nadir Alvarez a a Institute of Biology, University of Neuchâtel, Rue Emile-Argand 11, CH-2009 Neuchâtel, Switzerland b Molecular Systematics Section, Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, United Kingdom c Department of Botany, Smithsonian Institution, National Museum of Natural History, NHB-166, Washington, DC 20560, USA d Missouri Botanical Garden, PO Box 299, 63166-0299, St. Louis, MO, USA e Conservatoire et Jardin botaniques de la ville de Genève, ch. de l’Impératrice 1, CH-1292 Chambésy, Switzerland f Department of Botany, Stockholm University, SE-10691, Stockholm, Sweden g School of Marine and Tropical Biology, James Cook University, PO Box 6811, Cairns, Qld 4870, Australia h Department of Biodiversity and Conservation, Real Jardin Botanico – CSIC, Plaza de Murillo 2, 28014 Madrid, Spain article info abstract Article history: The economically important soapberry family (Sapindaceae) comprises about 1900 species mainly found Received 21 May 2008 in the tropical regions of the world, with only a few genera being restricted to temperate areas. The inf- Revised 27 November 2008 rafamilial classification of the Sapindaceae and its relationships to the closely related Aceraceae and Hip- Accepted 23 January 2009 pocastanaceae – which have now been included in an expanded definition of Sapindaceae (i.e., subfamily Available online 30 January 2009 Hippocastanoideae) – have been debated for decades. -

Lose the Plot: Cost-Effective Survey of the Peak Range, Central Queensland
Lose the plot: cost-effective survey of the Peak Range, central Queensland. Don W. Butlera and Rod J. Fensham Queensland Herbarium, Environmental Protection Agency, Mt Coot-tha Botanic Gardens, Mt Coot-tha Road, Toowong, QLD, 4066 AUSTRALIA. aCorresponding author, email: [email protected] Abstract: The Peak Range (22˚ 28’ S; 147˚ 53’ E) is an archipelago of rocky peaks set in grassy basalt rolling-plains, east of Clermont in central Queensland. This report describes the flora and vegetation based on surveys of 26 peaks. The survey recorded all plant species encountered on traverses of distinct habitat zones, which included the ‘matrix’ adjacent to each peak. The method involved effort comparable to a general flora survey but provided sufficient information to also describe floristic association among peaks, broad habitat types, and contrast vegetation on the peaks with the surrounding landscape matrix. The flora of the Peak Range includes at least 507 native vascular plant species, representing 84 plant families. Exotic species are relatively few, with 36 species recorded, but can be quite prominent in some situations. The most abundant exotic plants are the grass Melinis repens and the forb Bidens bipinnata. Plant distribution patterns among peaks suggest three primary groups related to position within the range and geology. The Peak Range makes a substantial contribution to the botanical diversity of its region and harbours several endemic plants among a flora clearly distinct from that of the surrounding terrain. The distinctiveness of the range’s flora is due to two habitat components: dry rainforest patches reliant upon fire protection afforded by cliffs and scree, and; rocky summits and hillsides supporting xeric shrublands. -
No. 38 List of Plant Diseases in American Samoa 2002
Land Grant Technical Report No. 38 List of Plant Diseases in American Samoa 2002 Fred Brooks, Plant Pathologist Land Grant Technical Report No. 44, American Samoa Community College Land Grant Program, Aug. 2002. This work was funded in part by Hatch grants SAM-015 and -020, United States Department of Agriculture, Cooperative State Research, Extension, and Education Service (CSREES) and administered by American Samoa Community College. The author bears full responsibility for its content. The author wishes to acknowledge O.C. Steele for his assistance in plant identifications, the Land Grant Forestry crew for their work on the brown root rot survey, and M.A. Schmaedick for his continued support. For more information on this publication, please contact: Fred Brooks, Plant Pathologist American Samoa Community College Land Grant Program Pago Pago, AS 96799 Tel. (684) 699-1394-1575 Fax (684) 699-5011 e-mail <[email protected]> Title page: cordana leaf spot of banana caused by Cordana musae. TABLE OF CONTENTS Page Introduction ............................................................................................................................................... iv About this text ........................................................................................................................................... vi Host-pathogen index .................................................................................................................................. 1 Pathogen-host index ................................................................................................................................. -

Telopea · Escholarship.Usyd.Edu.Au/Journals/Index.Php/TEL · ISSN 0312-9764 (Print) · ISSN 2200-4025 (Online)
Volume 6(4): 541-562 T elopea Publication Date: 1 July 1996 . , . _ . neRoyal dx.doi.org/io.775i/teiopeai9963023 Journal ot Plant Systematics “ 2 ™ plantnet.rbgsyd.nsw.gov.au/Telopea · escholarship.usyd.edu.au/journals/index.php/TEL · ISSN 0312-9764 (Print) · ISSN 2200-4025 (Online) Mabberley, Plant introduction and hybridisation in colonial NSW 541 Plant introduction and hybridisation in colonial New South Wales: the work of John Carne Bidwill, Sydney's first director D.J. Mabberley Abstract Mabberley, D.J. (Department of Plant Sciences, University of Oxford, Oxford 0X 1 3PN; and Rijksherbarium, University of Leiden) 1996. Plant introduction and hybridisation in colonial New South Wales: the work of John Carne Bidwill, Sydney's first director. Telopea 6(4): 541-562. A brief biography of J.C. Bidwill, the first Director of the Sydney Gardens, based in part on previously unpublished manuscript sources preserved at Royal Botanic Gardens Kew and in the Mitchell Library Sydney, is presented. Bidwill's scientific impact is assessed and there is an appendix of plants named after him; the hitherto unplaced Bidwillia is perhaps referable to Trachyandra (Asphodelaceae). Introduction 'He is, besides being an excellent botanist, a man of general science, a very skillful horticulturist' — William Macarthur on J.C. Bidwill, 17 September 1847 (Macarthur Papers 37(B) Sir William Macarthur Letterbook 4 viii 1844-7 vi 1850 f. 296, A2933-2 Mitchell Library). In celebrating Lawrie Johnson, here his interest in the history of botany, it is perhaps of some value to examine the career of one of his predecessors as Director of the Sydney Gardens — the first holder of that title, John Carne Bidwill (1815-1853) — as it highlights a number of features of nineteenth-century colonial life and attitudes. -

Flora of Australia, Volume 25, Melianthaceae to Simaroubaceae
FLORA OF AUSTRALIA Volume 25 Melianthaceae to Simaroubaceae This volume was published before the Commonwealth Government moved to Creative Commons Licensing. © Commonwealth of Australia 1985. This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced or distributed by any process or stored in any retrieval system or data base without prior written permission from the copyright holder. Requests and inquiries concerning reproduction and rights should be addressed to: [email protected] FLORA OF AUSTRALIA Volume 25 of Flora of Australia contains 7 families of plants. The largest is Sapindaceae, with 30 genera and 193 species. Many of these are rainforest plants of Queensland and New South Wales, but a number occur elsewhere in Australia. The family contains the large genus Dodonaea (native hops), which occurs widely in drier regions. Also in Volume 25 is Anacardiaceae, with 9 genera and 13 species in Australia. These arc mostly tropical plants but include several trees naturalised in southern regions. The other families are Simaroubaceae (4 genera, with 5 native species and 1 naturalised species), Burseraceae (2 genera, 5 native species), Melianthaceae (1 genus, 2 naturalised species), Akaniaceae (1 native species) and Aceraceae (1 naturalised species). In all, the volume contains 48 genera and 221 species. The volume includes descriptions, keys for identification, notes and maps on distribution, and bibliographic information. A number of species are illustrated by line drawings or colour photographs. -
NSW Rainforest Trees Part V
This document has been scanned from hard-copy archives for research and study purposes. Please note not all information may be current. We have tried, in preparing this copy, to make the content accessible to the widest possible audience but in some cases we recognise that the automatic text recognition maybe inadequate and we apologise in advance for any inconvenience this may cause. ODe 176.1 RESEARCH NOTE No. 32 PUBLISHED 1977 N.S.W. RAINFOREST TREES PART V FAMlLIES SAPINDACEAE AND AKANIACEAE AUTHOR A. G. FLOYD, M.Se.For., Dip.For. (Research Centre, Coffs Harbour Jetty, N.S.W.) FORESTRY COMMISSION OF NEW SOUTH WALES SYDNEY, 1977 G 34911-1. INTRODUCTION This is the fifth in a series of research notes describing the rainforest trees of N.S.W. Previous publications are- Research Note No. 3 (1960) N.S.W. Rainforest Trees. Part I, Family Lauraceae. A. G. Floyd and H. C. Hayes. Research Note No. 7 (1961) N.S.W. Rainforest Trees. PartIl, Families Capparidaceae, Escalloniaceae, Pittosporaceae, Cunoniaceae, Davidsoniaceae. A. G. Floyd and H. C. Hayes. Research Note No. 28 (1973) N.S.W. Rainforest Trees. Part Ill, Family Myrtaceae. A. G. Floyd. Research Note No. 29 (1976) N.S.W. Rainforest Trees. Part IV, Family Rutaceae. A. G. Floyd. Research Note No. 34 (1977) N.S.W. Rainforest Trees. Part VI, Families Podocarpaceae, Araucariaceae, Cupressaceae, Fagaceae, Ulmaceae, Moraceae, Urticaceae. A. G. Floyd. Each of the thirty-one species of the families Sapindaceae and Akaniaceae have been described with the emphasis on field characteristics. The diagnostic features are shown in italics. -
Ash Island Plant Species List
Ash Island Plant Species List Lowland KWRP Family Botanical Name Common name Woodland Floodplain + Nursery Rainforest Malvaceae Abutilon oxycarpum Lantern Bush 1 Fabaceae Acacia falciformis 1 Fabaceae Acacia floribunda Sunshine Wattle 1 1 1 Fabaceae Acacia implexa Hickory Wattle 1 1 1 Fabaceae Acacia longifolia Sydney Golden Wattle 1 1 Fabaceae Acacia maidenii Maidens Wattle 1 1 Myrtaceae Acmena smithii Lillypilly 1 1 Rutaceae Acronychia oblongifolia Lemon Aspen 1 1 Adiantaceae Adiantum aethiopicum Rough Maidenhair 1 Adiantaceae Adiantum formosum Maidenhair 1 Adiantaceae Adiantum hispidulum Rough Maidenhair Fern 1 Sapindaceae Alectryon subcinereus Wild Quince 1 1 Casuarinaceae Allocasuarina paludosa Swamp She-Oak 1 1 Araceae Alocasia brisbanensis Cunjevoi 1 1 Rhamnaceae Alphitonia excelsa Red Ash 1 1 Amaranthaceae Alternanthera denticulate Lesser Joyweed 1 1 Amaranthaceae Amaranthus sp. Amaranth Loranthaceae Amyema congener 1 Loranthaceae Amyema gaudichaudii 1 Loranthaceae Amyema pendulum 1 Loranthaceae Amyema sp. Mistletoe Cunoniaceae Aphanopetalum resinosum Gum Vine 1 1 Anthericaceae Arthropodium sp. Vanilla Lily 1 1 Aspleniaceae Asplenium australasicum Bird’s Nest Fern 1 Aspleniaceae Asplenium flabellifolium Necklace Fern 1 Chenopodiaceae Atriplex cinerea 1 Sterculiaceae Brachychiton populneus Kurrajong 1 1 1 Euphorbiaceae Breynia oblongifolia Coffee Bush 1 1 Myrtaceae Callistemon salignus White Bottlebrush 1 1 1 Convolvulaceae Calystegia marginata 1 Rubiaceae Canthium coprosmoides Coast Canthium 1 Capparaceae Capparis arborea Native -
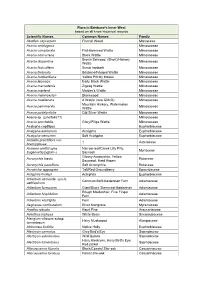
Flora in Brisbane's Inner West Based on All Know Historical Records
Flora in Brisbane's Inner West based on all know historical records Scientific Names Common Names Family Abutilon oxycarpum Flannel Weed Malvaceae Acacia amblygona Mimosaceae Acacia complanata Flat-stemmed Wattle Mimosaceae Acacia concurrens Black Wattle Mimosaceae Brown Salwood, (Short)/Hickory Acacia disparrima Mimosaceae Wattle Acacia fasiculifera Scrub Ironbark Mimosaceae Acacia fimbriata Brisbane/Fringed Wattle Mimosaceae Acacia hubbardiana Yellow Prickly Moses Mimosaceae Acacia leiocalyx Early Black Wattle Mimosaceae Acacia macradenia Zigzag Wattle Mimosaceae Acacia maidenii Maiden's Wattle Mimosaceae Acacia melanoxylon Blackwood Mimosaceae Acacia muellerana A Wattle (rare GMcD) Mimosaceae Mountain Hickory, Watermelon Acacia penninervis Mimosaceae Wattle Acacia podalyriifolia Qld Silver Wattle Mimosaceae Acacia sp. (juncifolia??) Mimosaceae Acacia spectabilis Glory/Piliga Wattle Mimosaceae Acalypha capillipes Euphorbiaceae Acalypha eremorum Acalypha Euphorbiaceae Acalypha nemorum Soft Acalypha Euphorbiaceae Acmella grandiflora var. ? Asteraceae brachyglossa Acmena smithii syns. Narrow-leaf/Creek Lilly Pilly, Myrtaceae Eugenia/Syzygium s. Satinash Glossy Acronychia, Yellow Acronychia laevis Rutaceae Boxwood, Hard Aspen Acronychia pauciflora Soft Acronychia Rutaceae Acrotriche aggregata Tall/Red Groundberry Epacridaceae Actephila lindleyi Actephila Euphorbiaceae Adiantum atroviride, syn A. Common/Soft Maidenhair Fern Adiantaceae aethiopicum Adiantum formosum Giant/Black Stemmed Maidenhair Adiantaceae Rough Maidenhair, Five Finger -

Phylogeny and Circumscription of Sapindaceae Revisited: Molecular Sequence Data, Morphology and Biogeography Support Recognition of a New Family, Xanthoceraceae
Plant Ecology and Evolution 143 (2): 148–159, 2010 doi:10.5091/plecevo.2010.437 REGULAR PAPER Phylogeny and circumscription of Sapindaceae revisited: molecular sequence data, morphology and biogeography support recognition of a new family, Xanthoceraceae Sven Buerki1, Porter P. Lowry II2,3,*, Nadir Alvarez4, Sylvain G. Razafimandimbison5, Philippe Küpfer6 & Martin W. Callmander2,7 1Department of Biodiversity and Conservation, Real Jardin Botanico, CSIC, Plaza de Murillo 2, ES-28014 Madrid, Spain 2Missouri Botanical Garden, P.O. Box 299, St. Louis, MO 63166-0299, U.S.A. 3Muséum National d’Histoire Naturelle, Case Postale 39, 57 rue Cuvier, FR-75231 05 CEDEX, Paris, France 4Department of Ecology and Evolution, Biophore, University of Lausanne, CH-1015 Lausanne, Switzerland 5Department of Botany, Bergius Foundation, SE-10691, Stockholm University, Stockholm, Sweden 6Institute of Biology, University of Neuchâtel, Rue Emile-Argand 11, CH-2000 Neuchâtel, Switzerland 7Conservatoire et Jardin botaniques de la ville de Genève, ch. de l’Impératrice 1, CH-1292 Chambésy, Switzerland *Author for correspondence: [email protected] Background and aims – Recent studies have adopted a broad definition of Sapindaceae that includes taxa traditionally placed in Aceraceae and Hippocastanaceae, achieving monophyly but yielding a family difficult to characterize and for which no obvious morphological synapomorphy exists. This expanded circumscription was necessitated by the finding that the monotypic, temperate Asian genus Xanthoceras, historically placed in Sapindaceae tribe Harpullieae, is basal within the group. Here we seek to clarify the relationships of Xanthoceras based on phylogenetic analyses using a dataset encompassing nearly ¾ of sapindaceous genera, comparing the results with information from morphology and biogeography, in particular with respect to the other taxa placed in Harpullieae.