Recovery Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species Status Assessment Report for the Barrens Darter (Etheostoma Forbesi)
Species Status Assessment Report for the Barrens Darter (Etheostoma forbesi) Version 2.0 Acknowledgements: This Species Status Assessment would not have been possible without the research and assistance of Dr. Richard Harrington, Yale University Department of Ecology and Evolutionary Biology, Dr. Hayden Mattingly and his students, Tennessee Tech University School of Environmental Studies, Dr. John Johansen, Austin Peay State University Department of Biology, and Mark Thurman, Tennessee Wildlife Resources Agency. 1 TABLE OF CONTENTS Chapter 1: Introduction ............................................................................................................... 3 Chapter 2: Biology and Life History ........................................................................................... 4 Taxonomy ................................................................................................................................ 4 Genetic Diversity ..................................................................................................................... 5 Morphological Description ...................................................................................................... 5 Habitat ..................................................................................................................................... 6 Lifecycle .................................................................................................................................. 7 Population Needs .................................................................................................................... -

Escribe Agenda Package
Board of Directors Meeting #7/19 was held at TRCA Head Office, on Friday, July 26, 2019. The Chair, Jennifer Innis, called the meeting to order at 9:33 a.m. PRESENT Jennifer Innis Chair Paul Ainslie Member Kevin Ashe Member Shelley Carroll Member Ronald Chopowick Member Joanne Dies Member Jennifer Drake Member Paula Fletcher Member Jack Heath Vice-Chair Gord Highet Member Linda Jackson Member Heidi Karst Member Maria Kelleher Member Cynthia Lai Member Mike Layton Member Basudeb Mukherjee Member Michael Palleschi Member Steve Pellegrini Member Anthony Perruzza Member Gino Rosati Member Connie Tang Member ABSENT David Barrow Member Dipika Damerla Member Chris Fonseca Member James Pasternak Member Jason Runtas Member Rowena Santos Member Estair Van Wagner Member The Chair recited the Acknowledgement of Indigenous Territory. RES.#A140/19 - MINUTES OF MEETING #6/19, HELD ON JUNE 21, 2019 Moved by: Michael Palleschi Seconded by: Paula Fletcher THAT the Board of Directors approves the Minutes of Meeting #7/19, held on Friday, June 21, 2019. CARRIED Section I – Items for Board of Directors Action RES.#A141/19 - REQUEST TO DELEGATE PERMIT APPROVAL AND CANCELLATION OF AUGUST 9, 2019 EXECUTIVE COMMITTEE MEETING The August 9, 2019 Executive Committee is proposed to be cancelled. Staff request the Board of Directors to delegate approval authority of all permits in the entire jurisdiction originally scheduled for the August 9, 2019 meeting to staff. Moved by: Linda Jackson Seconded by: Steve Pellegrini WHEREAS as a matter of new business at the July -

Native Freshwater Mussels
Native Freshwater Mussels Freshwater mussels, sometimes called clams, have always been and continue to be, an important food source for muskrat, minks, FACT SHEET raccoons, otters, fishes, and some birds such as herons. Historically, written by John Tautin Native Americans not only ate mussels but also used the shells for utensils, tools, and to make jewelry. Between the late 1800s and mid-1900s, shells were harvested to supply a multi- fluted shell million dollar pearl button industry. However, with the Lasmigona costata invention and widespread use of plastics during the 1940-50s, the pearl button industry collapsed. But by the 1950s the Japanese found a new use for mussel shells in cultured pearl production. e shells are cut and finished into beads and inserted into oysters to serve as nuclei for pearls. Still today, thousands of tons of mussel shells (especially Washboard, Mapleleaf, and ree-ridge mussels) are exported from the United States to Japan for this purpose. Elktoe Worldwide, there are about one thousand species of mussels. Mussels Alasmidonta marginata can be found on every continent but Antarctica. While the entire continent of Europe only has eight different species of mussels, there are twenty-five different species of mussels in French Creek, and about three-hundred in the United States. ese important animals are threatened, however. Today, over half of the species of mussel in the Midwest are threatened or endangered. In the French Creek watershed, thirteen species of mussels are listed as endan- gered or threatened in Pennsylvania. Four species (the northern riffleshell, kidney shell and clubshell, rayed bean, and snuffbox) are endangered at the federal level. -

Fish Inventory at Stones River National Battlefield
Fish Inventory at Stones River National Battlefield Submitted to: Department of the Interior National Park Service Cumberland Piedmont Network By Dennis Mullen Professor of Biology Department of Biology Middle Tennessee State University Murfreesboro, TN 37132 September 2006 Striped Shiner (Luxilus chrysocephalus) – nuptial male From Lytle Creek at Fortress Rosecrans Photograph by D. Mullen Table of Contents List of Tables……………………………………………………………………….iii List of Figures………………………………………………………………………iv List of Appendices…………………………………………………………………..v Executive Summary…………………………………………………………………1 Introduction…………………………………………………………………...……..2 Methods……………………………………………………………………………...3 Results……………………………………………………………………………….7 Discussion………………………………………………………………………….10 Conclusions………………………………………………………………………...14 Literature Cited…………………………………………………………………….15 ii List of Tables Table1: Location and physical characteristics (during September 2006, and only for the riverine sites) of sample sites for the STRI fish inventory………………………………17 Table 2: Biotic Integrity classes used in assessing fish communities along with general descriptions of their attributes (Karr et al. 1986) ………………………………………18 Table 3: List of fishes potentially occurring in aquatic habitats in and around Stones River National Battlefield………………………………………………………………..19 Table 4: Fish species list (by site) of aquatic habitats at STRI (October 2004 – August 2006). MF = McFadden’s Ford, KP = King Pond, RB = Redoubt Brannan, UP = Unnamed Pond at Redoubt Brannan, LC = Lytle Creek at Fortress Rosecrans……...….22 Table 5: Fish Species Richness estimates for the 3 riverine reaches of STRI and a composite estimate for STRI as a whole…………………………………………………24 Table 6: Index of Biotic Integrity (IBI) scores for three stream reaches at Stones River National Battlefield during August 2005………………………………………………...25 Table 7: Temperature and water chemistry of four of the STRI sample sites for each sampling date…………………………………………………………………………….26 Table 8 : Total length estimates of specific habitat types at each riverine sample site. -

Tennessee's Extinct Species
Tennessee's Extinct Species The following species Birds: once occurred in Carolina parakeet Conuropsis carolinensis Ectopistes migratorius Tennessee and are now Passenger pigeon believed to be extinct. Mammals: Following this list are two Eastern elk species descriptions-one Fishes: describing the Carolina Harelip sucker parakeet and another describing the extinct Mussels: Acornshell Epioblasma haysiana freshwater mussels Angled riffleshell Epioblasma biemarginata of Tennessee. Cumberland leafshell Epioblasma stewardsoni Leafshell Epioblasma flexuosa Narrowcat's paw Epioblasma lenoir Rough rockshell Quadrula tuberosa Round combshell Epioblasma personata Sugarspoon Epioblasma arcaeformis Tennessee riffleshell Epioblasma propinqua Carolina Parakeet Status Habitat The Carolina parakeet is an The Carolina parakeet was found Learn rrwreabout extinct species. in riverine forests, cypress swamps, Tennessee's diverse and other woodlands over much of Description the Eastern and Midwest Regions of ecosyster.n3.Su~ort The Carolina parakeet was a the United States. It was the only conservation in your small parrot, about 12inches in parrot native to the United States. community and state! length. Its head was lemon yellow, The parakeets rested at night in with an orange forehead and cheeks. groups, with as many as 30 birds The rest of its body was green. Its sleeping inside one hollowtree, while legs and beak were pale pinkish- others would hang on the outside. white. These curious birds lived and Nests were placed in hollowtrees, traveled in flocks. and three to five white eggs were laid. Up to 50 nests were often crowded into one tree. Role in the Ecosystem Carolina parakeets enjoyed a variety of different foods-apples, peaches, mulberries, pecans, grapes, dogwood fruit, and grains. -

C:\Fish\Eastern Sand Darter Sa.Wpd
EASTERN SAND DARTER STATUS ASSESSMENT Prepared by: David Grandmaison and Joseph Mayasich Natural Resources Research Institute University of Minnesota 5013 Miller Trunk Highway Duluth, MN 55811-1442 and David Etnier Ecology and Evolutionary Biology University of Tennessee 569 Dabney Hall Knoxville, TN 37996-1610 Prepared for: U.S. Fish and Wildlife Service Region 3 1 Federal Drive Fort Snelling, MN 55111 January 2004 NRRI Technical Report No. NRRI/TR-2003/40 DISCLAIMER This document is a compilation of biological data and a description of past, present, and likely future threats to the eastern sand darter, Ammocrypta pellucida (Agassiz). It does not represent a decision by the U.S. Fish and Wildlife Service (Service) on whether this taxon should be designated as a candidate species for listing as threatened or endangered under the Federal Endangered Species Act. That decision will be made by the Service after reviewing this document; other relevant biological and threat data not included herein; and all relevant laws, regulations, and policies. The result of the decision will be posted on the Service's Region 3 Web site (refer to: http://midwest.fws.gov/eco_serv/endangrd/lists/concern.html). If designated as a candidate species, the taxon will subsequently be added to the Service's candidate species list that is periodically published in the Federal Register and posted on the World Wide Web (refer to: http://endangered.fws.gov/wildlife.html). Even if the taxon does not warrant candidate status it should benefit from the conservation recommendations that are contained in this document. ii TABLE OF CONTENTS DISCLAIMER................................................................... -

Part IV: Scoring Criteria for the Index of Biotic Integrity to Monitor
Part IV: Scoring Criteria for the Index of Biotic Integrity to Monitor Fish Communities in Wadeable Streams in the Coosa and Tennessee Drainage Basins of the Ridge and Valley Ecoregion of Georgia Georgia Department of Natural Resources Wildlife Resources Division Fisheries Management Section 2020 Table of Contents Introduction………………………………………………………………… ……... Pg. 1 Map of Ridge and Valley Ecoregion………………………………..……............... Pg. 3 Table 1. State Listed Fish in the Ridge and Valley Ecoregion……………………. Pg. 4 Table 2. IBI Metrics and Scoring Criteria………………………………………….Pg. 5 References………………………………………………….. ………………………Pg. 7 Appendix 1…………………………………………………………………. ………Pg. 8 Coosa Basin Group (ACT) MSR Graphs..………………………………….Pg. 9 Tennessee Basin Group (TEN) MSR Graphs……………………………….Pg. 17 Ridge and Valley Ecoregion Fish List………………………………………Pg. 25 i Introduction The Ridge and Valley ecoregion is one of the six Level III ecoregions found in Georgia (Part 1, Figure 1). It is drained by two major river basins, the Coosa and the Tennessee, in the northwestern corner of Georgia. The Ridge and Valley ecoregion covers nearly 3,000 square miles (United States Census Bureau 2000) and includes all or portions of 10 counties (Figure 1), bordering the Piedmont ecoregion to the south and the Blue Ridge ecoregion to the east. A small portion of the Southwestern Appalachians ecoregion is located in the upper northwestern corner of the Ridge and Valley ecoregion. The biotic index developed by the GAWRD is based on Level III ecoregion delineations (Griffith et al. 2001). The metrics and scoring criteria adapted to the Ridge and Valley ecoregion were developed from biomonitoring samples collected in the two major river basins that drain the Ridge and Valley ecoregion, the Coosa (ACT) and the Tennessee (TEN). -

The Monitoring Handbook of Large Freshwater Mussels
The thick shelled river mussel (Unio crassus) brings LIFE+ back to rivers [UC4LIFE] Målarmusslans återkomst… ACTION A1 DELIVERABLE: “The Monitoring Handbook” The monitoring handbook of large freshwater mussels Jakob Bergengren1, Ted von Proschwitz2, Stefan Lundberg3, Håkan Söderberg4, Oskar Norrgrann4, Martin Österling5 and Ivan Olsson6 County Administrative Board of 1Jönköping 4Västernorrland and 6Skåne, The Natural History Museum in 2Gothenburg and 3Stockholm, and 5Karlstad university and County Administrative Board of Scania6 Action A.1 Deliverable” The monitoring Handbook” Abstract This handbook will be used as part the Life-project “The thick shelled river mussel (Unio crassus) brings LIFE+ back to rivers” [UC4LIFE] monitoring programme to ensure adequate and consistent sampling strategies and techniques amongst the sampling crew. Naturally, the handbook describes sampling strategies and techniques, not only designated for the LIFE- project, but constitute the basis for mussel sampling generally according to standard protocol. Thus, this handbook is based on results from former studies and experiences by the authors and the results have been published previously in different formats. The handbook demonstrate suitable sampling techniques which will be used to survey variables which include mussel population size, density and individual age/size before and after restoration measures (Actions C.1 and C.3 in this project) at the twelve project sites that are planned for in this project. Also, this handbook is relevant for additional project-actions, such as the planning work (Action A.3), Host-fish mapping (Action C.1), Re-introduction and allocation (Action C.3), including all information activities (Actions D.1 - D.7). 1 Action A.1 Deliverable” The monitoring Handbook” Background and objectives Nine species of mussels from the group ”large freshwater mussels” are currently found in Swedish waters. -

Restoring the Endangered Oyster Mussel (Epioblasma Capsaeformis) to the Upper Clinch River, Virginia: an Evaluation of Population Restoration Techniques Caitlin S
RESEARCH ARTICLE Restoring the endangered oyster mussel (Epioblasma capsaeformis) to the upper Clinch River, Virginia: an evaluation of population restoration techniques Caitlin S. Carey1,2,3,JessW.Jones4, Robert S. Butler5, Eric M. Hallerman6 From 2005 to 2011, the federally endangered freshwater mussel Epioblasma capsaeformis (oyster mussel) was reintroduced at three sites in the upper Clinch River, Virginia, using four release techniques. These release techniques were (1) translocation of adults (site 1, n = 1418), (2) release of laboratory-propagated sub-adults (site 1, n = 2851), (3) release of 8-week-old laboratory-propagated juveniles (site 2, n = 9501), and (4) release of artificially infested host fishes (site 3, n = 1116 host fishes). These restoration efforts provided a unique research opportunity to compare the effectiveness of techniques used to reestablish populations of extirpated and declining species. We evaluated the relative success of these four population restoration approaches via monitoring at each release site (2011–2012) using systematic 0.25-m2 quadrat sampling to estimate abundance and post-release survival. Abundances of translocated adult and laboratory-propagated sub-adult E. capsaeformis at site 1 ranged 577–645 and 1678–1700 individuals, respectively, signifying successful settlement and high post-release survival. Two untagged individuals (29.1 and 27.3 mm) were observed, indicating that recruitment is occurring at site 1. No E. capsaeformis were found at sites where 8-week-old laboratory-propagated juveniles (site 2) and artificially infested host fishes (site 3) were released. Our results indicate that translocations of adults and releases of laboratory-propagated sub-adults were the most effective population restoration techniques for E. -

Guiding Species Recovery Through Assessment of Spatial And
Guiding Species Recovery through Assessment of Spatial and Temporal Population Genetic Structure of Two Critically Endangered Freshwater Mussel Species (Bivalvia: Unionidae) Jess Walter Jones ( [email protected] ) United States Fish and Wildlife Service Timothy W. Lane Virginia Department of Game and Inland Fisheries N J Eric M. Hallerman Virginia Tech: Virginia Polytechnic Institute and State University Research Article Keywords: Freshwater mussels, Epioblasma brevidens, E. capsaeformis, endangered species, spatial and temporal genetic variation, effective population size, species recovery planning, conservation genetics Posted Date: March 16th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-282423/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/28 Abstract The Cumberlandian Combshell (Epioblasma brevidens) and Oyster Mussel (E. capsaeformis) are critically endangered freshwater mussel species native to the Tennessee and Cumberland River drainages, major tributaries of the Ohio River in the eastern United States. The Clinch River in northeastern Tennessee (TN) and southwestern Virginia (VA) harbors the only remaining stronghold population for either species, containing tens of thousands of individuals per species; however, a few smaller populations are still extant in other rivers. We collected and analyzed genetic data to assist with population restoration and recovery planning for both species. We used an 888 base-pair sequence of the mitochondrial NADH dehydrogenase 1 (ND1) gene and ten nuclear DNA microsatellite loci to assess patterns of genetic differentiation and diversity in populations at small and large spatial scales, and at a 9-year (2004 to 2013) temporal scale, which showed how quickly these populations can diverge from each other in a short time period. -
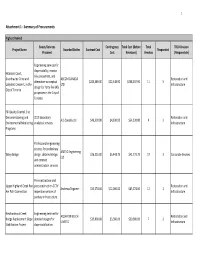
Attachment 1 Summary of Procurements.Pdf
1 Attachment 1 ‐ Summary of Procurements Highest Ranked Goods/Services Contingency Total Cost (Before Total TRCA Division Project Name Awarded Bidder Contract Cost Responded Procured Cost Revisions) Vendors (Responsible) Engineering services for slope stability, erosion Eldorado Court, risk assessment, and Grandravine Drive and AECOM CANADA Restoration and alternative conceptual $225,489.00 $22,548.90 $248,037.90 11 5 Ladyshot Crescent, in the LTD. Infrastructure design for forty‐five (45) City of Toronto properties in the City of Toronto. Fill Quality Control, Site Decommissioning and 2019 laboratory Restoration and ALS Canada Ltd $49,300.00 $4,930.00 $54,230.00 4 3 Environmental Monitoring analytical services. Infrastructure Programs Professional engineering services for preliminary AMTEC Engineering Wiley Bridge design, detailed design $36,325.00 $5,448.75 $41,773.75 17 3 Corporate Services Ltd. and contract administration services. Pre‐construction and Upper Highland Creek Pan post‐construction CCTV Restoration and Andrews Engineer $33,274.00 $12,000.00 $45,274.00 12 1 Am Path Connection inspection services of Infrastructure sanitary infrastructure. Newtonbrook Creek Engineering services for AQUAFOR BEECH Restoration and Bridge Replacement Slope detailed designs for $35,800.00 $3,580.00 $39,380.00 7 2 LIMITED Infrastructure Stabilization Project slope stabilization. 2 Attachment 1 ‐ Summary of Procurements Highest Ranked Goods/Services Contingency Total Cost (Before Total TRCA Division Project Name Awarded Bidder Contract Cost Responded Procured Cost Revisions) Vendors (Responsible) Engineering services for Grey Abbey Ravine Slope AQUAFOR BEECH Restoration and development of detailed $59,160.00 $5,916.00 $65,076.00 5 1 Stabilization Project LIMITED Infrastructure designs. -

Identifying Freshwater Mussels (Unionoida)
Identifying freshwater mussels (Unionoida) and parasitic glochidia larvae from host fish gills: a molecular key to the North and Central European species Alexandra Zieritz1, Bernhard Gum1, Ralph Kuehn2 &JuergenGeist1 1Aquatic Systems Biology Unit, Department of Ecology and Ecosystem Management, Technische Universitat¨ Munchen,¨ Muhlenweg¨ 22, 85354 Freising, Germany 2Molecular Zoology Unit, Chair of Zoology, Department of Animal Science, Technische Universitat¨ Munchen,¨ Hans-Carl-von-Carlowitz-Platz 2, 85354 Freising, Germany Keywords Abstract Host–parasite interactions, morphological variability, PCR-RFLP, species identification, Freshwater mussels (order Unionoida) represent one of the most severely endan- Unionidae, wildlife management. gered groups of animals due to habitat destruction, introduction of nonnative species, and loss of host fishes, which their larvae (glochidia) are obligate parasites Correspondence on. Conservation efforts such as habitat restoration or restocking of host popu- Juergen Geist, Aquatic Systems Biology Unit, lations are currently hampered by difficulties in unionoid species identification Department of Ecology and Ecosystem by morphological means. Here we present the first complete molecular identifi- Management, Technische Universitat¨ Munchen,¨ Muhlenweg¨ 22, 85354 Freising, cation key for all seven indigenous North and Central European unionoid species Germany. Tel: +49 (0)8161 713767; and the nonnative Sinanodonta woodiana, facilitating quick, low-cost, and reliable Fax: +49 (0)8161 713477; identification of adult and larval specimens. Application of this restriction frag- E-mail: [email protected] ment length polymorphisms (RFLP) key resulted in 100% accurate assignment of 90 adult specimens from across the region by digestion of partial ITS-1 (where ITS Received: 2 December 2011; Revised: 26 is internal transcribed spacer) polymerase chain reaction (PCR) products in two to January 2012; Accepted: 6 February 2012 four single digestions with five restriction endonucleases.