Nitrogen Retention and Removal in Sub-Tropical Riparian Zones
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

40736 Waterways and Coastal Management Strategy 2011
Section 7 Maps Sunshine Coast Waterways and Coastal Management Strategy 2011-2021 53 54 andCoastal ManagementStrategy2011-2021 Sunshine CoastWaterways Map 7.1 Sunshine Coast Catchments Major Catchments Noosa River Maroochy River Pumicestone Passage Sunshine Coast Catchments Mary River Stanley River Mooloolah River Water Bodies (see note) Watercourses (Order 5-8) 02461 km 1:220,000 Note A comprehensive map of constructed water bodies under Council management will be developed during implementation of the Strategy. Lake Document Path: W:\Common\Geo\Projects\140140_Strategy_Amendments\Maps\Waterways_Strategy\Map7_1_MajorCatchments_20141110_LK007 Data Source Weyba All catchment, stream and waterbodies datasets internal to the Sunshine Coast Council boundary have been derived by the Sunshine Coast Council. Catchment and stream datasets external to the Sunshine Coast Council boundary is a combination of Sunshine Coast Council and the Department of Environment and Resource Management datasets Disclaimer While every care is taken to ensure the accuracy of this product, neither the Sunshine Coast Council nor the State of Queensland makes any representations or warranties about its accuracy, reliability, completeness or suitability for any particular purpose and disclaims all responsibility and all liability (including without limitation, liability in negligence) for all expenses, losses, damages (including indirect or consequential damage) and costs that may occur as a result of the product being inaccurate or incomplete Eumundi in any way or -

Restricted Water Ski Areas in Queensland
Restricted Water Ski areas in Queensland Watercourse Date of Gazettal Any person operating a ship towing anyone by a line attached to the ship (including for example a person water skiing or riding on a toboggan or tube) within the waters listed below endangers marine safety. Brisbane River 20/10/2006 South Brisbane and Town Reaches of the Brisbane River between the Merivale Bridge and the Story Bridge. Burdekin River, Charters Towers 13/09/2019 All waters of The Weir on the Burdekin River, Charters Towers. Except: • commencing at a point on the waterline of the eastern bank of the Burdekin River nearest to location 19°55.279’S, 146°16.639’E, • then generally southerly along the waterline of the eastern bank to a point nearest to location 19°56.530’S, 146°17.276’E, • then westerly across Burdekin River to a point on the waterline of the western bank nearest to location 19°56.600’S, 146°17.164’E, • then generally northerly along the waterline of the western bank to a point on the waterline nearest to location 19°55.280’S, 146°16.525’E, • then easterly across the Burdekin River to the point of commencement. As shown on the map S8sp-73 prepared by Maritime Safety Queensland (MSQ) which can be found on the MSQ website at www.msq.qld.gov.au/s8sp73map and is held at MSQ’s Townsville Office. Burrum River .12/07/1996 The waters of the Burrum River within 200 metres north from the High Water mark of the southern river bank and commencing at a point 50 metres downstream of the public boat ramp off Burrum Heads Road to a point 200 metres upstream of the upstream boundary of Lions Park, Burrum Heads. -

Maroochy River Environmental Values and Water Quality
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! M A R O O C H Y R I V E R , I N C L U D I N G A L L T R I B U T A R I E S O F ! T H E R I V E R ! ! M A R O O C H Y R I V E R , I N C L U D I N G A L L T R I B U T A R I E S O F T H E R I V E R ! ! ! ! ! ! ! ! ! ! ! ! Part of Basin 141 ! ! ! ! ! ! ! ! ! ! ! 152°50'E 153°E ! 153°10'E ! ! -

Land Cover Change in the South East Queensland Catchments Natural Resource Management Region 2010–11
Department of Science, Information Technology, Innovation and the Arts Land cover change in the South East Queensland Catchments Natural Resource Management region 2010–11 Summary The woody vegetation clearing rate for the SEQ region for 10 2010–11 dropped to 3193 hectares per year (ha/yr). This 9 8 represented a 14 per cent decline from the previous era. ha/year) 7 Clearing rates of remnant woody vegetation decreased in 6 5 2010-11 to 758 ha/yr, 33 per cent lower than the previous era. 4 The replacement land cover class of forestry increased by 3 2 a further 5 per cent over the previous era and represented 1 Clearing Rate (,000 26 per cent of the total woody vegetation 0 clearing rate in the region. Pasture 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 remained the dominant replacement All Woody Clearing Woody Remnant Clearing land cover class at 34 per cent of total clearing. Figure 1. Woody vegetation clearing rates in the South East Queensland Catchments NRM region. Figure 2. Woody vegetation clearing for each change period. Great state. Great opportunity. Woody vegetation clearing by Woody vegetation clearing by remnant status tenure Table 1. Remnant and non-remnant woody vegetation clearing Table 2. Woody vegetation clearing rates in the South East rates in the South East Queensland Catchments NRM region. Queensland Catchments NRM region by tenure. Woody vegetation clearing rate (,000 ha/yr) of Woody vegetation clearing rate (,000 ha/yr) on Non-remnant Remnant -

Surface Water Ambient Network (Water Quality) 2020-21
Surface Water Ambient Network (Water Quality) 2020-21 July 2020 This publication has been compiled by Natural Resources Divisional Support, Department of Natural Resources, Mines and Energy. © State of Queensland, 2020 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence, visit https://creativecommons.org/licenses/by/4.0/. The information contained herein is subject to change without notice. The Queensland Government shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. Summary This document lists the stream gauging stations which make up the Department of Natural Resources, Mines and Energy (DNRME) surface water quality monitoring network. Data collected under this network are published on DNRME’s Water Monitoring Information Data Portal. The water quality data collected includes both logged time-series and manual water samples taken for later laboratory analysis. Other data types are also collected at stream gauging stations, including rainfall and stream height. Further information is available on the Water Monitoring Information Data Portal under each station listing. -

Queensland Water Quality Guidelines 2009
Queensland Water Quality Guidelines 2009 Prepared by: Environmental Policy and Planning, Department of Environment and Heritage Protection © State of Queensland, 2013. Re-published in July 2013 to reflect machinery-of-government changes, (departmental names, web addresses, accessing datasets), and updated reference sources. No changes have been made to water quality guidelines. The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 3.0 Australia (CC BY) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. For more information on this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Disclaimer This document has been prepared with all due diligence and care, based on the best available information at the time of publication. The department holds no responsibility for any errors or omissions within this document. Any decisions made by other parties based on this document are solely the responsibility of those parties. Information contained in this document is from a number of sources and, as such, does not necessarily represent government or departmental policy. If you need to access this document in a language other than English, please call the Translating and Interpreting Service (TIS National) on 131 450 and ask them to telephone Library Services on +61 7 3170 5470. This publication can be made available in an alternative format (e.g. -
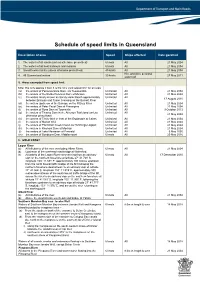
Schedule of Speed Limits in Queensland
Schedule of speed limits in Queensland Description of area Speed Ships affected Date gazetted 1. The waters of all canals (unless otherwise prescribed) 6 knots All 21 May 2004 2. The waters of all boat harbours and marinas 6 knots All 21 May 2004 3. Smooth water limits (unless otherwise prescribed) 40 knots All 21 May 2004 Hire and drive personal 4. All Queensland waters 30 knots 27 May 2011 watercraft 5. Areas exempted from speed limit Note: this only applies if item 3 is the only valid speed limit for an area (a) the waters of Perserverance Dam, via Toowoomba Unlimited All 21 May 2004 (b) the waters of the Bjelke Peterson Dam at Murgon Unlimited All 21 May 2004 (c) the waters locally known as Sandy Hook Reach approximately Unlimited All 17 August 2010 between Branyan and Tyson Crossing on the Burnett River (d) the waters upstream of the Barrage on the Fitzroy River Unlimited All 21 May 2004 (e) the waters of Peter Faust Dam at Proserpine Unlimited All 21 May 2004 (f) the waters of Ross Dam at Townsville Unlimited All 9 October 2013 (g) the waters of Tinaroo Dam in the Atherton Tableland (unless Unlimited All 21 May 2004 otherwise prescribed) (h) the waters of Trinity Inlet in front of the Esplanade at Cairns Unlimited All 21 May 2004 (i) the waters of Marian Weir Unlimited All 21 May 2004 (j) the waters of Plantation Creek known as Hutchings Lagoon Unlimited All 21 May 2004 (k) the waters in Kinchant Dam at Mackay Unlimited All 21 May 2004 (l) the waters of Lake Maraboon at Emerald Unlimited All 6 May 2005 (m) the waters of Bundoora Dam, Middlemount 6 knots All 20 May 2016 6. -

Principles and Tools for Protecting Australian Rivers
Principles and Tools for Protecting Australian Rivers N. Phillips, J. Bennett and D. Moulton The National Rivers Consortium is a consortium of policy makers, river managers and scientists. Its vision is to achieve continuous improvement in the health of Australia’s rivers. The role of the consortium is coordination and leadership in river restoration and protection, through sharing and enhancing the skills and knowledge of its members. Partners making a significant financial contribution to the National Rivers Consortium and represented on the Board of Management are: Land & Water Australia Murray–Darling Basin Commission Water and Rivers Commission, Western Australia CSIRO Land and Water Published by: Land & Water Australia GPO Box 2182 Canberra ACT 2601 Telephone: (02) 6257 3379 Facsimile: (02) 6257 3420 Email: [email protected] WebSite: www.lwa.gov.au © Land & Water Australia Disclaimer: The information contained in this publication has been published by Land & Water Australia to assist public knowledge and discussion and to help improve the sustainable management of land, water and vegetation. When technical information has been prepared by or contributed by authors external to the Corporation, readers should contact the author(s), and conduct their own enquiries, before making use of that information. Publication data: ‘Principles and Tools for Protecting Australian Rivers’, Phillips, N., Bennett, J. and Moulton, D., Land & Water Australia, 2001. Authors: Phillips, N., Bennett, J. and Moulton, D., Queensland Environmental Protection -

Ship Operations and Activities on the Maroochy River, Final Report to The
Ship operations and activities on the Maroochy River Final Report to the General Manager Final Report to the General Manager, Transport and Main Roads, July 2011 2 of 134 Document control sheet Contact for enquiries and proposed changes If you have any questions regarding this document or if you have a suggestion for improvements, please contact: Contact officer Peter Kleinig Title Area Manager (Sunshine Coast) Phone 07 5477 8425 Version history Version no. Date Changed by Nature of amendment 0.1 03/03/11 Peter Kleinig Initial draft 0.2 09/03/11 Peter Kleinig Minor corrections throughout document 0.3 10/03/11 Peter Kleinig Insert appendices and new maps 0.4 14/03/11 Peter Kleinig Updated recommendations and appendix 1 0.5 13/07/11 Peter Kleinig Updated following meeting 0.6 25/07/11 Peter Kleinig Inserted new maps 1.0 28/07/11 Peter Kleinig Final draft 1.1 28/07/11 Peter Kleinig Final Final Report to the General Manager, Transport and Main Roads, July 2011 3 of 134 Document sign off The following officers have approved this document. Customer Name Captain Richard Johnson Position Regional Harbour Master (Brisbane) Signature Date Reference Group chairperson Name John Kavanagh Position Director (Maritime Services) Signature Date The following persons have endorsed this document. Reference Group members Name Glen Ferguson Position Vice President, Maroochy River Water Ski Association Signature Date Name Graeme Shea Position Representative, Residents of Cook Road, Bli Bli Signature Date Name John Smallwood Position Representative, Sunshine -

40736 Waterways and Coastal Management Strategy 2011
Sunshine Coast Waterways and Coastal Management Strategy 2011-2021 August 2014 edition Our waterways - valued, healthy, enjoyed. For further information www.sunshinecoast.qld.gov.au (07) 5475 7272 Sunshine Coast Council™ is a registered trademark of Sunshine Coast Regional Council. © Sunshine Coast Regional Council 2009-current. Adopted by Council February 2011. Revised August 2014, due to Sunshine Coast Local Government Area boundary amendments. Acknowledgements Sunshine Coast Regional Council acknowledges the Traditional Owners of land across the Sunshine Coast and recognises their rich culture and intrinsic connection to the land and sea that stretches back over thousands of years. Council also wishes to thank all interested stakeholders for their valuable contributions towards the development of the Sunshine Coast Waterways and Coastal Management Strategy 2011–2021. Disclaimer Information contained in this document is based on available information at the time of writing. All figures and diagrams are indicative only and should be referred to as such. This is a strategic document which deals with technical matters in a summary way only. Council or its officers accept no responsibility for any loss occasioned to any person acting or refraining from acting in reliance upon any material contained in this document. 2 Sunshine Coast Waterways and Coastal Management Strategy 2011-2021 Table of contents 1 Executive Summary 44 Challenges 26 2 Background 8 4.1 Accommodating population 28 2.1 Waterways and coastal 10 growth and demand foreshores -

Maroochy River Environmental Values and Water Quality Objectives Basin No
Environmental Protection (Water) Policy 2009 Maroochy River environmental values and water quality objectives Basin No. 141 (part), including all tributaries of the Maroochy River July 2010 Prepared by: Water Quality & Ecosystem Health Policy Unit Department of Environment and Resource Management © State of Queensland (Department of Environment and Resource Management) 2010 The Department of Environment and Resource Management authorises the reproduction of textual material, whole or part, in any form, provided appropriate acknowledgement is given. This publication is available in alternative formats (including large print and audiotape) on request. Contact (07) 322 48412 or email <[email protected]> July 2010 Document Ref Number Main parts of this document and what they contain • Scope of waters covered Introduction • Key terms / how to use document (section 1) • Links to WQ plan (map) • Mapping / water type information • Further contact details • Amendment provisions • Source of EVs for this document Environmental Values • Table of EVs by waterway (EVs - section 2) - aquatic ecosystem - human use • Any applicable management goals to support EVs • How to establish WQOs to protect Water Quality Objectives all selected EVs (WQOs - section 3) • WQOs in this document, for - aquatic ecosystem EV - human use EVs • List of plans, reports etc containing Ways to improve management actions relevant to the water quality waterways in this area (section 4) • Definitions of key terms including an Dictionary explanation table of all (section 5) environmental values • An accompanying map that shows Accompanying WQ Plan water types, levels of protection and (map) other information contained in this document iii CONTENTS 1 INTRODUCTION ............................................................................................................................. 1 1.1 WATERS TO WHICH THIS DOCUMENT APPLIES ............................................................................. -

The 2053 South Queensland Flood
THE 2053 SOUTH QUEENSLAND FLOOD Only Young Water Professionals Need Attend ABSTRACT A ‘roadshow’ of flood engineers in the early 1980s ‘prophesised’ the flooding that occurred in South East Queensland (SE QLD) during 2011 to 2015. The events of 2011 to 2015 may have given increased credibility to a long-held notion that SE QLD may be subject to a 40-year flood cycle. This paper examines the data on flooding within the ten major SE QLD catchments, bounded by three mountain ranges and comprising more than 63,000 km2. The purpose was to test the existence and the strength of any regularity to flooding in this well-populated region of Australia. The approach identifies the flooding that occurred during predetermined five-year periods that were 35 years apart – the 40-year cycle – and the flooding that occurred outside of these nominated 5-year periods. The basic statistics were weighted for the length of flood record, catchment area, ranking of flood, and a combination of these factors. The results indicate that 69-87% of major floods in SE QLD have occurred within a set of five-year periods exactly 35 years apart. No explanation is offered at this stage of the research. The implications for traditional hydrologic methods are outlined. Keywords: Flooding, flood cycles, regularity, flood frequency INTRODUCTION The early years of the 1970s in South East Queensland was a period of multiple flooding events, remembered mostly for the January 1974 flooding of Brisbane. The following decade was one that experienced widespread estuarine development, where State and Local Government concerns that the development be above the 1in100AEP flood on top of a cyclonic surge, with allowance for future water level rises due to global climatic change, engaged a community of engineers who specialised in relevant fields.