Modeling of Detached Solidification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Venona Special Studies
- 1 - Venona Project Special Studies Transcribed by Students of the Mercyhurst College Institute for Intelligence Studies Arranged by John Earl Haynes, Library of Congress, 2010 COVER NAMES IN NEW YORK TRAFFIC p. 2 UNIDENTIFIED COVER NAMES IN NEW YORK TRAFFIC p. 86 COVER NAMES IN SAN FRANCISCO TRAFFIC p. 92 COVER NAMES IN WASHINGTON TRAFFIC p. 123 ADDITIONAL COVERNAMES AND RELATED INFORMATION IN DIPLOMATIC TRAFFIC p. 127 REVISED TRANSLATION OF MESSAGE ON ANTENNA-LIBERAL'S WIFE ETHEL p. 135 THE COVERNAMES "ANTENNA" AND "LIBERAL" IN . MESSAGES p. 139 ESSAGES IN . INVOLVING THE COVERNAME"ENORMOZ" AND THE NAMES OF NUCLEAR PHYSICISTS, ETC. p. 147 UNDATED REPORT OF MEREDITH GARDNER p. 155 DEVELOPMENT OF THE “G--“HOMER” [“GOMER”] CASE p. 158 THE KOMAR (KRAVCHENKO) AFFAIR IN . MESSAGES p. 161 REVISED TRANSLATION OF TWO . MESSAGES ON CHANGES IN COVERNAMES p. 170 THE COVERNAME "KARAS" IN. TRAFFIC p. 178 THE COVERNAMES "TÉNOR", "BAS", AND "CHETÁ" (? IN . TRAFFIC p. 181 - 2 - Special Study Cover Names in New York Traffic - 3 - cover-name Message number Date Publication reference S/ or 3/NBF/ 19 N.Y. to M. 812 29053 JKI 06 T1022 1B-1910 0027A ABRAM N.Y. to M. 992 24063 JKR 14 T872√ 1B-7518 0005A JACK SOBLE 1086 06073 JKV 48 T873√ 2A-0011 1957 29113 NNNNNN T939√ 625 04054 JHD 48 T916√ 851 15064 JIJ 40 T10.1√ 1146 10084 JHM 41 T123√ 1251 02094 JHN 12 T301√ (to ChEKh) 0005B 1353 23094 JHO 42 T289√ 1449 12104 JIL 37 T106√ 1754 14124 JHZ 49 T6√ 48 11015 JHV 37 (NSA)T1941 AVGUR 2A-0013 1638 (AUGUR) N.Y. -

Lunar Life Sciences Payload Assessment
Lunar Surface Science Workshop 2020 (LPI Contrib. No. 2241) 5077.pdf LUNAR LIFE SCIENCES PAYLOAD ASSESSMENT. S. C. Sun1, F. Karouia2, M. P. Lera3, M. P. Parra1, H. E. Ray4, A. J. Ricco1, S. M. Spremo1. 1NASA Ames Research Center, 2Blue Marble Space Institute of Science, 3KBR, 4ASRC Federal Space and Defense, Inc. Introduction: The Moon provides a unique site to ISS, including systems that integrate into EXPRESS study living organisms. The fractional gravity and (EXpedite the PRocessing of ExperimentS for Space) unique radiation environment have similarities to Mars Racks or are external space exposure research facilities. and will help us understand how life will respond to These same systems can be the basis for future payload conditions on the red planet. Martian and lunar envi- systems for experiments to be performed beyond Low ronments can be simulated on the ground but not to high Earth Orbit. Such facilities would need to be adapted to fidelity. Altered gravity and increased radiation are dif- be compatible with the new research platforms and ficult to replicate simultaneously, which makes study- function in the harsher radiation environment found out- ing their combined effect difficult. The International side the magnetosphere. If Gateway and a lunar based- Space Station, and previously, the Space Shuttle, pro- lab could provide EXPRESS-compatible interfaces, lev- vided a microgravity environment, and could simulate eraging hardware developed for ISS would be more fea- fractional-g only via an onboard centrifuge. Because sible. the ISS and Space Shuttle orbits were within the Earth’s Gaps in Capabilities: Many of the payload systems magnetosphere, experiments on those platforms have that have been developed require human tending. -

18Th EANA Conference European Astrobiology Network Association
18th EANA Conference European Astrobiology Network Association Abstract book 24-28 September 2018 Freie Universität Berlin, Germany Sponsors: Detectability of biosignatures in martian sedimentary systems A. H. Stevens1, A. McDonald2, and C. S. Cockell1 (1) UK Centre for Astrobiology, University of Edinburgh, UK ([email protected]) (2) Bioimaging Facility, School of Engineering, University of Edinburgh, UK Presentation: Tuesday 12:45-13:00 Session: Traces of life, biosignatures, life detection Abstract: Some of the most promising potential sampling sites for astrobiology are the numerous sedimentary areas on Mars such as those explored by MSL. As sedimentary systems have a high relative likelihood to have been habitable in the past and are known on Earth to preserve biosignatures well, the remains of martian sedimentary systems are an attractive target for exploration, for example by sample return caching rovers [1]. To learn how best to look for evidence of life in these environments, we must carefully understand their context. While recent measurements have raised the upper limit for organic carbon measured in martian sediments [2], our exploration to date shows no evidence for a terrestrial-like biosphere on Mars. We used an analogue of a martian mudstone (Y-Mars[3]) to investigate how best to look for biosignatures in martian sedimentary environments. The mudstone was inoculated with a relevant microbial community and cultured over several months under martian conditions to select for the most Mars-relevant microbes. We sequenced the microbial community over a number of transfers to try and understand what types microbes might be expected to exist in these environments and assess whether they might leave behind any specific biosignatures. -

NASA Ames to Establish Nationwide Lunar Science Institute
November 2007 Worden gives upbeat message about future work for Ames BY JOHN BLUCK "We have switched material to In an upbeat talk to a crowd that phenolic impregnated carbon abla- filled the Ames main auditorium, tor (PICA), a (heat shield) material Ames Center Director S. Pete Worden developed here," Worden noted. His outlined an exciting future at Ames projected slide also listed Ames as that includes new work in exploration, leading PICA development and test- science and aeronautics -- each about a ing both for the Crew Exploration Ve- third of the center's efforts, he said. "I hicle, now called Orion, and the Mars have a gazillion charts to go through," Science Laboratory (MSL), which has photo by Eric James NASA he said. a planned launch date in fall 2009. His wide-ranging presentation Worden said that Ames' arc jets about Ames touched on moon explo- facility "a unique facility in the world." ration, a lunar institute, moon dust re- He added, "We want to upgrade search, heat shield work for spacecraft them." destined for the moon and Mars, a Mars sample "cache box" assignment, Life Sciences rising supercomputer capability, small "We are getting additional life Ames Center Director S. Pete Worden responds satellite work with a potential for support tasks assigned by Johnson to a question during the recent upbeat talk he many missions, increased astrobiology (and Marshall)," Worden said. "This is gave to the center about the future of Ames. work, growing cooperation among significant." continued on page 5 academia, and commercial partners and Ames and much more. -

6 FOTON RETRIEVABLE CAPSULES This Section Is Aimed at Providing New and Experienced Users with Basic Utilisation Information Regarding Foton Retrievable Capsules
6 FOTON RETRIEVABLE CAPSULES This section is aimed at providing new and experienced users with basic utilisation information regarding Foton retrievable capsules. It begins with an introduction to the Foton capsule. 6.1 Introduction to Foton Capsules 6.1.1 What Are Foton Capsules? Foton capsules (Figure 6-1 and Figure 6-2) are unmanned, retrievable capsules, derived from the design of the 1960’s Soviet Vostok manned spacecraft and the Zenit military reconnaissance satellite. These capsules are very similar to the Bion and Resurs-F satellites introduced by the Soviets in the 1970’s, for biological research and Earth natural resources investigation, respectively. The first Foton capsule was launched in 1985 as Cosmos 1645 and only with the fourth launch in 1988 was the spacecraft officially designated Foton (Foton-4). These capsules are launched into near-circular, low-earth orbits by a Soyuz-U rocket, providing researchers with gravity levels less than 10 -5 g, for missions lasting approximately 2 weeks. The earlier Foton missions were conceived primarily for materials science research, but later missions also began to include experiments in the fields of fluid physics, biology and radiation dosimetry. ESA’s participation in the Foton programme began in 1991 with a protein crystallisation experiment on-board Foton-7, followed by a further 35 experiments up to and including the Foton- 12 mission in 1999. In 2002, ESA provided a large number of experiments for the Foton-M1 mission (the first flight of an upgraded version of the Foton spacecraft). This mission ended in disaster when the Soyuz launcher rocket exploded shortly after lift-off due to a malfunction in one of its engines. -

Genesat (Launched Dec 2006), – Pre-Sat/Nanosail-D (Aug 2008) – Pharmasat (Launched May 2009), – O/OREOS (Planned May
National Aeronautics and Space Administration Free Flyer Utilization for Biology Research John W. Hines Chief Technologist, Engineering Directorate Technical Director, Nanosatellite Missions NASA-Ames Research Center NASA Applications of BioScience/BioTechnology HumanHuman ExplorationExploration EmphasisEmphasis FundamentalFundamental ExploratiExploratioonn Subsystems BiologyBiology Subsystems EmphasisEmphasis HumansHumans SmallSmall OrganismsOrganisms (Mice,(Mice, Rats) Rats) TiTissussue,e, O Orrgansgans MammalianMammalian CellsCells Human Health Emphasis ModelModel Organisms, BioMolecules Organisms, BioMolecules MicrobesMicrobes 2 4 Free-Flyer Utilization Free Flyer Features • Advantage: Relatively inexpensive means to increase number of flight opportunities • Capabilities: – Returnable capsule to small secondary non-recoverable satellites, and/or – In-situ measurement and control with autonomous sample management • Command and Control: Fully automated or uplinked command driven investigations. • Research data: Downlink and/or receipt of the samples • Collaborations: Interagency, academic, commercial and international Russian Free Flyers Early Free Flyers NASA Biosatellite I, II, 1966-67 NASA Biosatellite III, 1969 Nominal 3d flights Nominal 20d flight • Response to microgravity & • Spaceflight responses of non-human radiation: various biological species primates • Onboard radiation source Timeline of Russian-NASA Biology Spaceflights Collaborations Bion* Characteristics Bion Rationale • Increases access to space • Proven Platforms -
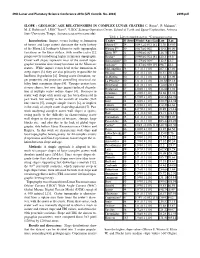
Slope - Geologic Age Relationships in Complex Lunar Craters C
49th Lunar and Planetary Science Conference 2018 (LPI Contrib. No. 2083) 2399.pdf SLOPE - GEOLOGIC AGE RELATIONSHIPS IN COMPLEX LUNAR CRATERS C. Rojas1, P. Mahanti1, M. S. Robinson1, LROC Team1, 1LROC Science Operation Center, School of Earth and Space Exploration, Arizona State University, Tempe, Arizona ([email protected]) Table 1: List of complex craters. *Copernican craters Introduction: Impact events leading to formation Crater D (km) Model Age (Ga) Lon Lat of basins and large craters dominate the early history Moore F* 24 0.041∓0.012 [8] 37.30 185.0 of the Moon [1] leading to kilometer scale topographic Wiener F* 30 0.017∓0.002 149.9740.90 variations on the lunar surface, with smaller crater [2], Klute W* 31 0.090∓0.007 216.7037.98 progressively introducing higher frequency topography. Necho* 37 0.080∓0.024 [8] 123.3 –5.3 Crater wall slopes represent most of the overall topo- Aristarchus* 40 0.175∓0.0095 312.5 23.7 graphic variation since many locations on the Moon are Jackson* 71 0.147∓0.038 [9] 196.7 22.1 craters. While impact events lead to the formation of McLaughlin 75 3.7∓0.1 [10] 267.1747.01 steep slopes [3], they are also primarily responsible for Pitiscus 80 3.8∓0.1 [10] 30.57 -50.61 landform degradation [4]. During crater formation, tar- Al-Biruni 80 3.8∓0.1 [10] 92.62 18.09 get properties and processes controlling structural sta- La Pérouse 80 3.6∓0.1 [10] -10.66 76.18 bility limit maximum slopes [4]. -

0418 Space Cluster Brochure FINAL
HARWELL SPACE MultidisciplinaryCLUSTER Innovation CONTENTS HARWELL FOREWORD CAMPUS 2 Harwell Campus 4 Success of the Harwell Space Cluster 6 Multidisciplinary Innovation 5,500people 8 Building the Harwell Space Cluster 9 Vision for the Future Harwell Campus is an exciting place to be, with cutting edge 10 UK Space Industry science facilities, major organisations and a great mix of companies from start-ups to multinationals. The Campus was quick to realise 12 Stakeholder Organisations £2+bnfacilities that it needed a mechanism to encourage collaboration, knowledge 20 Companies Driving Innovation at Harwell sharing and drive innovation, which led to the development of 40 Life at Harwell thematic Clusters. It started with the Harwell Space Cluster and now includes the HealthTec and EnergyTec Clusters. 42 Harwell Tomorrow 45 Contact I have watched Harwell Campus flourish over the last seven years, including the Harwell Space Cluster, which has grown to 80 organisations employing 800 people. I don’t expect there to be any let up in this growth and I look forward to the Campus changing, literally before my very eyes. SPACE I am really excited about the opportunities at the intersections between these Clusters, such as between the Space and HealthTec Clusters. Harwell CLUSTER Campus is able to demonstrate multidisciplinary innovation every day. There is no better way to really understand what is happening than to visit. I hope that you will do just that and that you will become part of the exciting future of the Harwell Space Cluster and help the UK reach organisations80 its goal of taking 10% of the global space market by 2030. -

Lingua Franca Nova English Dictionary
Lingua Franca Nova English Dictionary 16 October 2012 http://lfn.wikia.com/ http://webspace.ship.edu/cgboer/lfn/ http://purl.org/net/lfn/disionario/ 1 Lingua Franca Nova (LFN) is an auxiliary constructed language created by Dr C George Boeree of Shippensburg University, Pennsylvania. This is a printable copy of the master dictionary held online at http://purl.org/net/lfn/disionario/. A printable English–LFN dictionary can be downloaded from the same location. Abbreviations ABBR = abbreviation ADJ = adjective ADV = adverb BR = British English COMP = compound word (verb + noun) CONJ = conjunction DET = determiner INTERJ = interjection N = noun NUM = numeral PL = plural PREF = prefix PRENOM = prenominal (used before a noun) PREP = preposition PREVERB = preverbal (used before a verb) PRON = pronoun SUF = suffix US = American English V = verb VI = intransitive verb VT = transitive verb Indicators such as (o-i) and (e-u) mark words in which two vowels do not form a diphthong in normal pronunciation. 2 termination; aborta natural V miscarry; N miscarriage; A abortada ADJ abortive; ADV abortively; abortiste N abortionist; antiabortiste ADJ N antiabortionist A N A (letter, musical note) abracadabra! INTERJ abracadabra! hocus-pocus! a PREP at, in, on (point in space or time); to (movement); abrasa VT embrace, hug; clamp; N embrace, hug; abrasa toward, towards, in the direction of (direction); to ursin N bear hug; abrasable ADJ embraceable, (recipient) huggable; abrasador N clamp; abrasador fisada N vise a INTERJ ah, aha (surprise, sudden realization, -

Technologies for Exploration
SP-1254 SP-1254 TTechnologiesechnologies forfor ExplorationExploration T echnologies forExploration AuroraAurora ProgrammeProgramme Proposal:Proposal: Annex Annex DD Contact: ESA Publications Division c/o ESTEC, PO Box 299, 2200 AG Noordwijk, The Netherlands Tel. (31) 71 565 3400 - Fax (31) 71 565 5433 SP-1254 November 2001 Technologies for Exploration Aurora Programme Proposal: Annex D This report was written by the staff of ESA’s Directorate of Technical and Operational Support. Published by: ESA Publications Division ESTEC, PO Box 299 2200 AG Noordwijk The Netherlands Editor/layout: Andrew Wilson Copyright: © 2001 European Space Agency ISBN: 92-9092-616-3 ISSN: 0379-6566 Printed in: The Netherlands Price: €30 / 70 DFl technologies for exploration Technologies for Exploration Contents 1 Introduction 3 2 Exploration Milestones 3 3 Explorations Missions 3 4Technology Development and Associated Cost 5 5 Conclusion 6 Annex 1: Automated Guidance, Navigation & Control A1.1 and Mission Analysis Annex 2: Micro-Avionics A2.1 Annex 3: Data Processing and Communication Technologies A3.1 Annex 4: Entry, Descent and Landing A4.1 Annex 5: Crew Aspects of Exploration A5.1 Annex 6: In Situ Resource Utilisation A6.1 Annex 7: Power A7.1 Annex 8: Propulsion A8.1 Annex 9: Robotics and Mechanisms A9.1 Annex 10: Structures, Materials and Thermal Control A10.1 1 Table 1. Exploration Milestones for the Definition of Technology Readiness Requirements. 2005-2010 In situ resource utilisation/life support (ground demonstration) Decision on development of alternative -

Ages of Large Lunar Impact Craters and Implications for Bombardment During the Moon’S Middle Age ⇑ Michelle R
Icarus 225 (2013) 325–341 Contents lists available at SciVerse ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus Ages of large lunar impact craters and implications for bombardment during the Moon’s middle age ⇑ Michelle R. Kirchoff , Clark R. Chapman, Simone Marchi, Kristen M. Curtis, Brian Enke, William F. Bottke Southwest Research Institute, 1050 Walnut Street, Suite 300, Boulder, CO 80302, United States article info abstract Article history: Standard lunar chronologies, based on combining lunar sample radiometric ages with impact crater den- Received 20 October 2012 sities of inferred associated units, have lately been questioned about the robustness of their interpreta- Revised 28 February 2013 tions of the temporal dependance of the lunar impact flux. In particular, there has been increasing focus Accepted 10 March 2013 on the ‘‘middle age’’ of lunar bombardment, from the end of the Late Heavy Bombardment (3.8 Ga) until Available online 1 April 2013 comparatively recent times (1 Ga). To gain a better understanding of impact flux in this time period, we determined and analyzed the cratering ages of selected terrains on the Moon. We required distinct ter- Keywords: rains with random locations and areas large enough to achieve good statistics for the small, superposed Moon, Surface crater size–frequency distributions to be compiled. Therefore, we selected 40 lunar craters with diameter Cratering Impact processes 90 km and determined the model ages of their floors by measuring the density of superposed craters using the Lunar Reconnaissance Orbiter Wide Angle Camera mosaic. Absolute model ages were computed using the Model Production Function of Marchi et al. -

The Response of Bacillus Subtilis to Simulated Martian Conditions and to the Space Environment P
The response of Bacillus subtilis to simulated Martian conditions and to the space environment P. Rettberg (1), E. Rabbow (1), C. Panitz (2), G. Horneck (1), G. Reitz (1) 1. DLR, Institute of Aerospace Medicine, Radiation Biology Division, Köln, Germany 2. RWTH Aachen, Institute of Aviation Medicine, Aachen, Germany [email protected]; phone: +49 2203 6014637; fax: +49 2203 61970 The early histories of Mars and Earth show similarities during the period when life emerged on Earth. Thus a comparable early biological evolution might have taken place also on Mars. Several ongoing international space missions are especially de- signed to search for past or present life on Mars. In order to develop adequate instru- ments and methods for in situ life detection analysis and to avoid the contamination of Mars by terrestrial life forms introduced to it’s surface unintentionally, it is neces- sary to understand the potential and limits of life on Earth. The determination of the survival of microorganisms under the physical and chemical ‘extremes’ of Mars will provide detailed insights into the potential for contamination that will allow the de- velopment and improvement of planetary protection measures. Our knowledge about the occurrence of life, especially microbial life, on Earth has increased enormously in the last decades. Archaea, bacteria, and protista have been found living in many newly discovered extremely hostile habitats, which were regarded up to now as too harsh to harbor life. Whereas many newly discovered extremophile species are specialized to cope with one extreme environmental parameter like high or low temperature, high or low pH, high salt concentration, desiccation, high flux of ionizing or non-ionizing radiation, there are also long-known dormant stages of certain bacteria such as the Bacillus endospores, that are capable to withstand most of the environmental parame- ters on the surface of Mars like low average temperature, desiccation, CO2 dominated atmosphere, low pressure, high ionizing and UV radiation.