IVG Forest Conservation Report 5B: Giant Eucalypt Forests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Symposium Booklet
Date: Friday 18 March 2016 SYMPOSIUM BOOKLET Location: A one‐day symposium on eucalypt diversity The Royal Society of and conservation: scientific insights into the Victoria, future of Australian forests, woodlands and 8 La Trobe Street biota. Melbourne, Victoria Brought to you by: The Bjarne K Dahl Trust & The Royal Society of Victoria Welcome to the 2016 Conserving Eucalypts Symposium, brought to you by the Bjarne K Dahl Trust and the Royal Society of Victoria. We would like to thank the presenters for volunteering their time and wisdom to the day’s proceedings. Today’s symposium is part of National Eucalypt Day 2016 (#NED2016) celebrations promoted by the Bjarne K Dahl Trust. Further information can be found at dahltrust.org.au and at our respective Twitter sites @DahlTrust and @RoyalSocietyVic . Please write blog posts or Tweets about your symposium experience [respecting the rule of not using people’s names unless you have permission from them] and send a link to your post to @DahlTrust and @RoyalSocietyVic. Use #NED2016 as a tag to contribute to conversations around National Eucalypt Day 2016. Table of Contents Information for Attendees .............................................................................. 3 Program .......................................................................................................... 4 Abstracts ......................................................................................................... 5 Elective Field Trip: Mt Rothwell Biodiversity Interpretation Centre .............. -
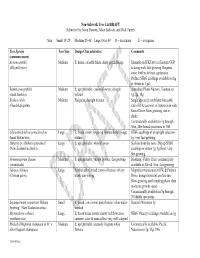
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

Seed Ecology Iii
SEED ECOLOGY III The Third International Society for Seed Science Meeting on Seeds and the Environment “Seeds and Change” Conference Proceedings June 20 to June 24, 2010 Salt Lake City, Utah, USA Editors: R. Pendleton, S. Meyer, B. Schultz Proceedings of the Seed Ecology III Conference Preface Extended abstracts included in this proceedings will be made available online. Enquiries and requests for hardcopies of this volume should be sent to: Dr. Rosemary Pendleton USFS Rocky Mountain Research Station Albuquerque Forestry Sciences Laboratory 333 Broadway SE Suite 115 Albuquerque, New Mexico, USA 87102-3497 The extended abstracts in this proceedings were edited for clarity. Seed Ecology III logo designed by Bitsy Schultz. i June 2010, Salt Lake City, Utah Proceedings of the Seed Ecology III Conference Table of Contents Germination Ecology of Dry Sandy Grassland Species along a pH-Gradient Simulated by Different Aluminium Concentrations.....................................................................................................................1 M Abedi, M Bartelheimer, Ralph Krall and Peter Poschlod Induction and Release of Secondary Dormancy under Field Conditions in Bromus tectorum.......................2 PS Allen, SE Meyer, and K Foote Seedling Production for Purposes of Biodiversity Restoration in the Brazilian Cerrado Region Can Be Greatly Enhanced by Seed Pretreatments Derived from Seed Technology......................................................4 S Anese, GCM Soares, ACB Matos, DAB Pinto, EAA da Silva, and HWM Hilhorst -

Failing to Conserve Leadbeater's Possum and Its Mountain Ash Forest
CORE Metadata, citation and similar papers at core.ac.uk Provided by The Australian National University Failing to conserve Leadbeater’s Possum and its Mountain Ash forest habitat David Blair1, David Lindenmayer1 &Lachlan McBurney1 1Fenner School of Environment and Society, The Australian National University, Canberra, ACT 2601 Corresponding author: [email protected] The conservation of the Critically Endangered Leadbeater’s Possum Gymnobelideus leadbeateri in Victoria’s Mountain Ash Eucalyptus regnans forests is one of the most controversial native mammal conservation issues in Australia. Much of the controversy results from long-running conflicts between the demands of the native forest logging industry and associated impacts on Leadbeater’s Possum and its Mountain Ash forest habitat. Here we argue that despite a legislative obligation to protect Leadbeater’s Possum and some limited recent improvements in management, conservation efforts for the species have gone backwards over the past decade. The key problems we identify include that the Victorian Government has: (1) maintained levels of wood production that are too high given the amount of the forest estate that was burned in 2009, (2) failed to substitute clearfell logging practices with more ecologically-sensitive Variable Retention Harvesting Systems, (3) ignored the science (including by its own researchers) on the need for a large protected area for Leadbeater’s Possum, (4) altered key definitions such as those for mature trees and old growth that have substantially weakened the ability to protect Leadbeater’s Possum, and (5) overlooked the array of forest values beyond timber production (such as water and tourism) and which make a greater contribution to the economy. -

Pests, Diseases, and Aridity Have Shaped the Genome of Corymbia Citriodora
Lawrence Berkeley National Laboratory Recent Work Title Pests, diseases, and aridity have shaped the genome of Corymbia citriodora. Permalink https://escholarship.org/uc/item/5t51515k Journal Communications biology, 4(1) ISSN 2399-3642 Authors Healey, Adam L Shepherd, Mervyn King, Graham J et al. Publication Date 2021-05-10 DOI 10.1038/s42003-021-02009-0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California ARTICLE https://doi.org/10.1038/s42003-021-02009-0 OPEN Pests, diseases, and aridity have shaped the genome of Corymbia citriodora ✉ Adam L. Healey 1,2 , Mervyn Shepherd 3, Graham J. King 3, Jakob B. Butler 4, Jules S. Freeman 4,5,6, David J. Lee 7, Brad M. Potts4,5, Orzenil B. Silva-Junior8, Abdul Baten 3,9, Jerry Jenkins 1, Shengqiang Shu 10, John T. Lovell 1, Avinash Sreedasyam1, Jane Grimwood 1, Agnelo Furtado2, Dario Grattapaglia8,11, Kerrie W. Barry10, Hope Hundley10, Blake A. Simmons 2,12, Jeremy Schmutz 1,10, René E. Vaillancourt4,5 & Robert J. Henry 2 Corymbia citriodora is a member of the predominantly Southern Hemisphere Myrtaceae family, which includes the eucalypts (Eucalyptus, Corymbia and Angophora; ~800 species). 1234567890():,; Corymbia is grown for timber, pulp and paper, and essential oils in Australia, South Africa, Asia, and Brazil, maintaining a high-growth rate under marginal conditions due to drought, poor-quality soil, and biotic stresses. To dissect the genetic basis of these desirable traits, we sequenced and assembled the 408 Mb genome of Corymbia citriodora, anchored into eleven chromosomes. Comparative analysis with Eucalyptus grandis reveals high synteny, although the two diverged approximately 60 million years ago and have different genome sizes (408 vs 641 Mb), with few large intra-chromosomal rearrangements. -

Fire Management Newsletter: Eucalyptus: a Complex Challenge
Golden Gate National Recreation Area National Park Service U.S. Department of the Interior Point Reyes National Seashore EucalyptusEucalyptus A Complex Challenge AUSTRALIA FIRE MANAGEMENT, RESOURCE PROTECTION, AND THE LEGACY OF TASMANIAN BLUE GUM DURING THE AGE OF EXPLORATION, CURIOUS SPECIES dead, dry, oily leaves and debris—that is especially flammable. from around the world captured the imagination, desire and Carried by long swaying branches, fire spreads quickly in enterprising spirit of many different people. With fragrant oil and eucalyptus groves. When there is sufficient dead material in the massive grandeur, eucalyptus trees were imported in great canopy, fire moves easily through the tree tops. numbers from Australia to the Americas, and California became home to many of them. Adaptations to fire include heat-resistant seed capsules which protect the seed for a critical short period when fire reaches the CALIFORNIA Eucalyptus globulus, or Tasmanian blue gum, was first introduced crowns. One study showed that seeds were protected from lethal to the San Francisco Bay Area in 1853 as an ornamental tree. heat penetration for about 4 minutes when capsules were Soon after, it was widely planted for timber production when exposed to 826o F. Following all types of fire, an accelerated seed domestic lumber sources were being depleted. Eucalyptus shed occurs, even when the crowns are only subjected to intense offered hope to the “Hardwood Famine”, which the Bay Area heat without igniting. By reseeding when the litter is burned off, was keenly aware of, after rebuilding from the 1906 earthquake. blue gum eucalyptus like many other species takes advantage of the freshly uncovered soil that is available after a fire. -

Trees for Farm Forestry: 22 Promising Species
Forestry and Forest Products Natural Heritage Trust Helping Communities Helping Australia TREES FOR FARM FORESTRY: 22 PROMISING SPECIES Forestry and Forest Products TREES FOR FARM FORESTRY: Natural Heritage 22 PROMISING SPECIES Trust Helping Communities Helping Australia A report for the RIRDC/ Land & Water Australia/ FWPRDC Joint Venture Agroforestry Program Revised and Edited by Bronwyn Clarke, Ian McLeod and Tim Vercoe March 2009 i © 2008 Rural Industries Research and Development Corporation. All rights reserved. ISBN 1 74151 821 0 ISSN 1440-6845 Trees for Farm Forestry: 22 promising species Publication No. 09/015 Project No. CSF-56A The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. The Commonwealth of Australia does not necessarily endorse the views in this publication. -

Fungal Planet Description Sheets: 716–784 By: P.W
Fungal Planet description sheets: 716–784 By: P.W. Crous, M.J. Wingfield, T.I. Burgess, G.E.St.J. Hardy, J. Gené, J. Guarro, I.G. Baseia, D. García, L.F.P. Gusmão, C.M. Souza-Motta, R. Thangavel, S. Adamčík, A. Barili, C.W. Barnes, J.D.P. Bezerra, J.J. Bordallo, J.F. Cano-Lira, R.J.V. de Oliveira, E. Ercole, V. Hubka, I. Iturrieta-González, A. Kubátová, M.P. Martín, P.-A. Moreau, A. Morte, M.E. Ordoñez, A. Rodríguez, A.M. Stchigel, A. Vizzini, J. Abdollahzadeh, V.P. Abreu, K. Adamčíková, G.M.R. Albuquerque, A.V. Alexandrova, E. Álvarez Duarte, C. Armstrong-Cho, S. Banniza, R.N. Barbosa, J.-M. Bellanger, J.L. Bezerra, T.S. Cabral, M. Caboň, E. Caicedo, T. Cantillo, A.J. Carnegie, L.T. Carmo, R.F. Castañeda-Ruiz, C.R. Clement, A. Čmoková, L.B. Conceição, R.H.S.F. Cruz, U. Damm, B.D.B. da Silva, G.A. da Silva, R.M.F. da Silva, A.L.C.M. de A. Santiago, L.F. de Oliveira, C.A.F. de Souza, F. Déniel, B. Dima, G. Dong, J. Edwards, C.R. Félix, J. Fournier, T.B. Gibertoni, K. Hosaka, T. Iturriaga, M. Jadan, J.-L. Jany, Ž. Jurjević, M. Kolařík, I. Kušan, M.F. Landell, T.R. Leite Cordeiro, D.X. Lima, M. Loizides, S. Luo, A.R. Machado, H. Madrid, O.M.C. Magalhães, P. Marinho, N. Matočec, A. Mešić, A.N. Miller, O.V. Morozova, R.P. Neves, K. Nonaka, A. Nováková, N.H. -

Vicforests' Koala Management
VicForests Instruction Koala Management August 2015 1.0 Copyright © VicForests All rights reserved. No part of this document may be reproduced, stored in a retrieval system, or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise, without prior written permission of VicForests. Document Information General Information File Path : J:\VICFORESTS\PUBLIC\SUST. FOREST. MAN. SYSTEM\ FOREST OPERATIONS MANUAL\WRITTEN INSTRUCTIONS\APPROVED\VICFORESTS INSTRUCTION – KOALA MANAGEMENT RecFind Ref: Description: This document outlines VicForests process for managing Koalas Author: C. Powell Creation Date: July 2015 Procedure Owner(s): Conservation Biologist Current Version: 1.0 Copy Number: 1 Review Period: Last review date: 5/08/2015 Next review date: 5/08/2018 Revision History New Version Revision Date Author(s) Old Version Revision Notes Reviewers The following positions should review the instruction prior to any significant amendment being approved General Manager Planning Manager Forest Performance Approval Approver Position / Resolution Date Nathan Trushell General Manager, Planning Signature: Unless stamped ‘CONTROLLED COPY’ in red, all printed copies of this procedure are uncontrolled. The latest version can be found in VicForests SharePoint– Sustainable Forest Management System Version: 1.0 Last Updated: 5/08/15 Procedure Owner: Conservation Biologist Page 2 of 9 TABLE OF CONTENTS 1. Purpose .................................................................................................................................... -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

Evolutionary Relationships in Eucalyptus Sens. Lat. – a Synopsis
Euclid - Online edition Evolutionary relationships in Eucalyptus sens. lat. – a synopsis This article complements the introductory essay about eucalypts included in the "Learn about Eucalypts" section. Its aim is to provide an up-to-date account of the outcomes of research derived from different groups during the past 5 years relating to relationships within Eucalyptus s.s. As such it includes only those publications and hypotheses relating to higher level relationships of major groupings within the eucalypts. Some of the research reported below also provides insights into biogeographic relationships of the eucalypt group – in large part these are not the focus of this article and are not discussed in detail. Introduction The first comprehensive classification of the eucalypts was published by Blakely in 1934, in which he treated more than 600 taxa, building on earlier work of Maiden and Mueller. Blakely's classification remained the critical reference for Eucalyptus taxonomists for the next 37 years when a new but informal classification was published by Pryor and Johnson (1971). In this work the authors divided the genus into seven subgenera, and although of an informal nature, presented a system of great advance on Blakely's treatment. The small genus Angophora was retained. The next 20 years saw much debate about the naturalness of Eucalyptus and whether other genera should be recognized (e.g., Johnson 1987). Based on morphological data, Hill and Johnson in 1995 proposed a split in the genus and recognition of the genus Corymbia. This new genus of c. 113 species, comprised the ghost gums and the bloodwoods, and Hill and Johnson concluded that Corymbia is the sister group to Angophora, with the synapomorphy of the distinctive cap cells on bristle glands (Ladiges 1984) being unambiguous. -

Eucalyptus Plantations in the Bay Area
The History, Ecology and Future of Eucalyptus Plantations in the Bay Area Joe R. McBride Department of Landscape Architecture and Environmental Planning and Department of Environmental Science, Policy and Management University of California Berkeley, CA “The Eucalyptus seems an indispensable element of this State’s landscapes, as indigenously Californian as the redwoods, the poppy fields, the long white coastal beaches, the gleaming granite of the High Sierra.” H. Gilliam, 1965 Overview 1. History of eucalyptus in California 2. Characteristics of eucalyptus plantations 3. Modification of site conditions by eucalyptus 4. Eucalyptus forests as habitat for wildlife 5. Future of eucalyptus plantations in California Location of Eucalyptus Study Sites • Jack London State Park • Pt. Pinole • Tilden Park Angle Island • • Strawberry Canyon East Ft. Baker • • Redwood Park Lands End •• •Mills College Presidio • Chabot Park Burleigh Murray Ranch State Park • History • Initial Introduction • Planting during the 1870s • Planting from 1906-1913 • Planting in the latter half of the 20th century Initial Introduction of Eucalyptus to California Eucalyptus Planting in the 1870s Eucalyptus Planting 1906 -1912 Latter Half of the 20th Century Major Species of Eucalyptus Planted in California Blue Gum Red Gum Sugar Gum Red Ironbark Silver Dollar Lemon Scented Distribution of Blue Gum Eucalyptus Characteristics of Eucalyptus Plantations Structural Characteristics 80 Initial Spacing of Trees in Plantations (Angel Island State Park) Diameter Distribution of Eucalyptus