Tree Diseases of Eastern Canada
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annotated Check List and Host Index Arizona Wood
Annotated Check List and Host Index for Arizona Wood-Rotting Fungi Item Type text; Book Authors Gilbertson, R. L.; Martin, K. J.; Lindsey, J. P. Publisher College of Agriculture, University of Arizona (Tucson, AZ) Rights Copyright © Arizona Board of Regents. The University of Arizona. Download date 28/09/2021 02:18:59 Link to Item http://hdl.handle.net/10150/602154 Annotated Check List and Host Index for Arizona Wood - Rotting Fungi Technical Bulletin 209 Agricultural Experiment Station The University of Arizona Tucson AÏfJ\fOTA TED CHECK LI5T aid HOST INDEX ford ARIZONA WOOD- ROTTlNg FUNGI /. L. GILßERTSON K.T IyIARTiN Z J. P, LINDSEY3 PRDFE550I of PLANT PATHOLOgY 2GRADUATE ASSISTANT in I?ESEARCI-4 36FZADAATE A5 S /STANT'" TEACHING Z z l'9 FR5 1974- INTRODUCTION flora similar to that of the Gulf Coast and the southeastern United States is found. Here the major tree species include hardwoods such as Arizona is characterized by a wide variety of Arizona sycamore, Arizona black walnut, oaks, ecological zones from Sonoran Desert to alpine velvet ash, Fremont cottonwood, willows, and tundra. This environmental diversity has resulted mesquite. Some conifers, including Chihuahua pine, in a rich flora of woody plants in the state. De- Apache pine, pinyons, junipers, and Arizona cypress tailed accounts of the vegetation of Arizona have also occur in association with these hardwoods. appeared in a number of publications, including Arizona fungi typical of the southeastern flora those of Benson and Darrow (1954), Nichol (1952), include Fomitopsis ulmaria, Donkia pulcherrima, Kearney and Peebles (1969), Shreve and Wiggins Tyromyces palustris, Lopharia crassa, Inonotus (1964), Lowe (1972), and Hastings et al. -

Melampsora Medusae
European and Mediterranean Plant Protection Organization PM 7/93 (1) Organisation Europe´enne et Me´diterrane´enne pour la Protection des Plantes Diagnostics Diagnostic Melampsora medusae Specific scope Specific approval and amendment This standard describes a diagnostic protocol for Melampsora Approved in 2009–09. medusae1 Populus trichocarpa, and their interspecific hybrids (Frey et al., Introduction 2005). In Europe, most of the cultivated poplars are P. · eur- Melampsora medusae Thu¨men is one of the causal agents of americana (P. deltoides · P. nigra)andP. · interamericana poplar rust. The main species involved in this disease in Europe (P. trichocarpa · P. deltoides) hybrids. In addition, Shain (1988) on cultivated poplars are Melampsora larici-populina and showed evidence for the existence of two formae speciales within Melampsora allii-populina. All three species cause abundant M. medusae and named them M. medusae f. sp. deltoidae and uredinia production on leaves, which can lead to premature M. medusae f. sp. tremuloidae according to their primary host, defoliation and growth reduction. After several years of severe Populus deltoides and Populus tremuloides, respectively. Neither infection leading to repeated defoliation, the disease may predis- the EU directive, nor the EPPO make the distinction between pose the trees to dieback or even death for the younger trees. these two formae speciales but only refer to M. medusae.Not- Melampsora rust is a very common disease of poplar trees, which withstanding, since M. medusae was only reported in Europe on can cause severe economic losses in commercial poplar cultiva- poplars from the sections Aigeiros and Tacamahaca,andin tion because of the emergence and spread of new pathotypes of respect with the host specialization, it can be stated that only M. -

Cytospora Canker
report on RPD No. 604 PLANT April 1996 DEPARTMENT OF CROP SCIENCES DISEASE UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN CYTOSPORA OR LEUCOSTOMA CANKER OF SPRUCE Cytospora or Leucostoma canker, the most common and damaging disease of spruce, is caused by the fungus Leucocytospora kunzei, synonym Cytospora kunzei (teleomorph or sexual state Leucostoma kunzei, synonym Valsa kunzei). This canker occurs on several conifers from New England to the western United States. Colo- rado or Colorado blue (Picea pungens) and Norway spruce (Picea abies), used for ornament and in wind- breaks, are the species most commonly affected in Illinois. The disease has reached epidemic proportions on Engelmann spruce (Picea engelmannii) and Douglas- fir (Pseudotsuga menziesii) in the eastern Rocky Mountains due to a succession of dry years in the area. Other trees reported as susceptible to the disease are given in Table 1. Spruce trees less than 10 to 15 years old usually do not have Cytospora canker. In landscape nurseries, how- ever, small branches of young Colorado blue and oc- casionally white spruces may be killed. Three varieties of Leucostoma kunzei are recognized by some spec- ialists: var. piceae on spruces, var. superficialis on pines, and var. kunzei on other conifers. Figure 1. Colorado spruce affected by Cytospora Dead and dying branches call attention to Cytospora or canker. Leucostoma canker with older branches more suscep- tible than young ones. The fungus kills areas of bark, usually at the bases of small twigs and branches, creating elliptical to diamond-shaped lesions. If the lesions enlarge faster than the stem and girdle it, the portion beyond the canker also dies. -

Comparative and Population Genomics Landscape of Phellinus Noxius
bioRxiv preprint doi: https://doi.org/10.1101/132712; this version posted September 17, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Comparative and population genomics landscape of Phellinus noxius: 2 a hypervariable fungus causing root rot in trees 3 4 Chia-Lin Chung¶1,2, Tracy J. Lee3,4,5, Mitsuteru Akiba6, Hsin-Han Lee1, Tzu-Hao 5 Kuo3, Dang Liu3,7, Huei-Mien Ke3, Toshiro Yokoi6, Marylette B Roa3,8, Meiyeh J Lu3, 6 Ya-Yun Chang1, Pao-Jen Ann9, Jyh-Nong Tsai9, Chien-Yu Chen10, Shean-Shong 7 Tzean1, Yuko Ota6,11, Tsutomu Hattori6, Norio Sahashi6, Ruey-Fen Liou1,2, Taisei 8 Kikuchi12 and Isheng J Tsai¶3,4,5,7 9 10 1Department of Plant Pathology and Microbiology, National Taiwan University, Taiwan 11 2Master Program for Plant Medicine, National Taiwan University, Taiwan 12 3Biodiversity Research Center, Academia Sinica, Taipei, Taiwan 13 4Biodiversity Program, Taiwan International Graduate Program, Academia Sinica and 14 National Taiwan Normal University 15 5Department of Life Science, National Taiwan Normal University 16 6Department of Forest Microbiology, Forestry and Forest Products Research Institute, 17 Tsukuba, Japan 18 7Genome and Systems Biology Degree Program, National Taiwan University and Academia 19 Sinica, Taipei, Taiwan 20 8Philippine Genome Center, University of the Philippines, Diliman, Quezon City, Philippines 21 1101 -

Research Collection
Research Collection Doctoral Thesis Studies on Venturiaceae on Rosaceous plants Author(s): Menon, Radha Publication Date: 1956 Permanent Link: https://doi.org/10.3929/ethz-a-000092066 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss ETH Prom. Nr. 2585 B Studies on Venturiaceae on Rosaceous Plants THESIS PRESENTED TO THE SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH FOR THE DEGREE OF DOCTOR OF NATURAL SCIENCES BY RADHA MENON at CITIZEN OF Ser\ INDIA Accepted on the recommendation of Prof. Dr. E. Gaumann and Prof. Dr. A. Frey-Wyssling 19 5 6 Druck von A. W. Hayn's Erben, Berlin SO 36 Veroffentlicht in „Phytopathologische Zcitschrift" Band 27, Heft 2, Seite 117 bis 146 (1956) Verlag Paul Parey, Berlin und Hamburg From the Department of special Botany of the Swiss Federal Institute of Technology in Zurich Director: Prof. Dr. E. Gdumann Studies on Venturiaceae on Rosaceous Plants By Radha Menon With 10 Figures Contents: I. General Introduction. A. Venturiaceae. B. Venturiaceae on Rosaceae: 1) Venturia, 2) Coleroa, 3) Gibbera, 4) Xenomeris, 5) Apiosporina. — II. Experimental Part. A. Cultural Studies. B. Inoculation Experiments: 1) Introduction, 2) Inoculation Studies, 3) Results, 4) Conclusions. — III. Morphological and Cultural Studies. A. Genus Venturia: 1) Venturia inaequalis, 2) Venturia tomentosae, 3) Venturia pirina, 4) Venturia pruni-cerasi, 5) Venturia Mullcri, 6) Venturia potentillae, 7) Venturia palustris, 8) Venturia alchemillae. — Appendix: Fusicladium eriobotryae. — B. Genus Coleroa: Coleroa chac- tomium. — C. Genus Gibbera: Gibbera rosae. -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -

Diseases of Trees in the Great Plains
United States Department of Agriculture Diseases of Trees in the Great Plains Forest Rocky Mountain General Technical Service Research Station Report RMRS-GTR-335 November 2016 Bergdahl, Aaron D.; Hill, Alison, tech. coords. 2016. Diseases of trees in the Great Plains. Gen. Tech. Rep. RMRS-GTR-335. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 229 p. Abstract Hosts, distribution, symptoms and signs, disease cycle, and management strategies are described for 84 hardwood and 32 conifer diseases in 56 chapters. Color illustrations are provided to aid in accurate diagnosis. A glossary of technical terms and indexes to hosts and pathogens also are included. Keywords: Tree diseases, forest pathology, Great Plains, forest and tree health, windbreaks. Cover photos by: James A. Walla (top left), Laurie J. Stepanek (top right), David Leatherman (middle left), Aaron D. Bergdahl (middle right), James T. Blodgett (bottom left) and Laurie J. Stepanek (bottom right). To learn more about RMRS publications or search our online titles: www.fs.fed.us/rm/publications www.treesearch.fs.fed.us/ Background This technical report provides a guide to assist arborists, landowners, woody plant pest management specialists, foresters, and plant pathologists in the diagnosis and control of tree diseases encountered in the Great Plains. It contains 56 chapters on tree diseases prepared by 27 authors, and emphasizes disease situations as observed in the 10 states of the Great Plains: Colorado, Kansas, Montana, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming. The need for an updated tree disease guide for the Great Plains has been recog- nized for some time and an account of the history of this publication is provided here. -

Spruce Tree Challenge! Take a Walk in the Woods with This Self-Guided Scavenger Hunt to Learn More About White and Black Spruce Trees
Spruce Tree Challenge! Take a walk in the woods with this self-guided scavenger hunt to learn more about white and black spruce trees. Developed for use in Denali National Park and Preserve, but adaptable to interior Alaska. 1. Meet a spruce tree—make a new friend! Spruce trees are evergreen trees with needles and cones. Take a walk and choose a spruce tree to “meet”; find one with cones on it. Then make and record observa- tions to find out what kind of spruce tree you just met. Please be kind to your new friend; touch it gently and leave all cones and branches on the tree. But, feel free to shake hands with or give your tree a hug when introducing yourself! Find the large cones near the top of your tree. Draw or describe their shape. What kind of spruce is your new friend? Circle one. Picea glauca (white spruce)- long cylindrical cones on branch ends and clustered near the top of the tree; has no hairs on the back of its twigs (hint: look between the needles near the end of a branch). White spruce prefers well drained, upland sites. Picea mariana (black spruce) - small oval cones often located along trunk and at the top of the tree; tiny red-brown hairs on the backs of twigs between needles (try a camera zoom or hand lens to see). Black spruce prefer low lying areas with more moisture like bogs or near wetlands. Clipart courtesy FCIT If you gave your friend a name, what would it be? __________________________ Want to share a picture of you and your new friend? Or meet other spruce friends? Post on social media with #sprucetreechallenge 2. -
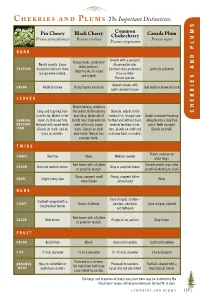
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

Sand Plums for Home and Commercial Production
Oklahoma Cooperative Extension Service HLA-6258 Sand Plums for Home and Commercial Production Beth McMahon Oklahoma Cooperative Extension Fact Sheets Research Assistant Oklahoma State University are also available on our website at: http://osufacts.okstate.edu Bruce Dunn Assistant Professor Geyer, 2010). Flowering will last for a couple of weeks and Oklahoma State University either red or yellow fruit will begin to form afterward. Ripening of the fruit occurs from June to early August and are either Sand plums, also known as Chickasaw plum, Cherokee yellow or a bright red. Both colors occur in the same areas plum, or Sandhill plum (Prunus angustifolia Marshall), are native of Oklahoma. Fruit size can range from ¼ inch to 1 inch. It fruit-producing shrubs or small trees in Oklahoma (Figure 1). is recommended that long sleeves be worn while collecting Use of sand plums range from cover for native bird species fruit since the plants may be thorny, depending upon how to making jams, jellies, and wine from the fruit. Commercial damaged they have been by deer and cattle in the past. desire in making jams and jellies has led to a rising interest in cultivating sand plums for home and orchard production. The purpose of this publication is to provide some basic knowledge Selecting Plants on how to identify, propagate, and grow your own sand plums. Besides selecting plants for fruit size and crop load, Sand plums range from 2 feet to 25 feet high, depend- you may also want to consider selecting plants that have ing upon soil and water conditions (Row and Geyer, 2010). -

Relationships Between Wood-Inhabiting Fungal Species
Silva Fennica 45(5) research articles SILVA FENNICA www.metla.fi/silvafennica · ISSN 0037-5330 The Finnish Society of Forest Science · The Finnish Forest Research Institute Relationships between Wood-Inhabiting Fungal Species Richness and Habitat Variables in Old-Growth Forest Stands in the Pallas-Yllästunturi National Park, Northern Boreal Finland Inari Ylläsjärvi, Håkan Berglund and Timo Kuuluvainen Ylläsjärvi, I., Berglund, H. & Kuuluvainen, T. 2011. Relationships between wood-inhabiting fungal species richness and habitat variables in old-growth forest stands in the Pallas-Yllästunturi National Park, northern boreal Finland. Silva Fennica 45(5): 995–1013. Indicators for biodiversity are needed for efficient prioritization of forests selected for conservation. We analyzed the relationships between 86 wood-inhabiting fungal (polypore) species richness and 35 habitat variables in 81 northern boreal old-growth forest stands in Finland. Species richness and the number of red-listed species were analyzed separately using generalized linear models. Most species were infrequent in the studied landscape and no species was encountered in all stands. The species richness increased with 1) the volume of coarse woody debris (CWD), 2) the mean DBH of CWD and 3) the basal area of living trees. The number of red-listed species increased along the same gradients, but the effect of basal area was not significant. Polypore species richness was significantly lower on western slopes than on flat topography. On average, species richness was higher on northern and eastern slopes than on western and southern slopes. The results suggest that a combination of habitat variables used as indicators may be useful in selecting forest stands to be set aside for polypore species conservation. -

Hypoxylon Canker
LANDOWNER UPDATE: CYTOSPORA CANKER Cytospora canker, caused by a fungal cuts to avoid accidentally spreading the pathogen known as Leucostoma kunzei, is disease. the most common disease of Norway spruce and Colorado blue spruce in Delaware. Spraying fungicides is not recommended for These trees are not native to Delaware but this disease. Some fungal material is located have been widely planted because they make internally and therefore will not be affected attractive yard specimens. by sprays. This disease usually affects older trees. Homeowners often notice that the needles on the lower branches of their trees turn brown (Figure 1), and eventually these branches die. Usually, branch dieback is accompanied by profuse resin flow from cankers on infected branches (Figure 2). Cankers are dead areas that sometimes have small black fungal fruiting bodies around their edges. If left untreated, the disease can progress up the tree as the fruiting bodies release spores during wet conditions. Figure 2. Resin oozing from a canker. Stress predisposes trees to infection. You can reduce stress by watering your trees during hot summer months without rain. Water at least once per week. Mulch around the base of trees to retain soil moisture. Avoid wounding trees with lawnmowers and weedeaters. Trees suffering from this disease, if left untreated, will often develop other, secondary problems with bark beetles, Figure 1. Dieback caused by Cytospora canker. spider mites, and diseases. It is important to address Cytospora canker on an ongoing The recommended treatments for this basis, through pruning and stress reduction, disease include pruning and stress reduction to help prevent these other problems.