What Types of Foods Are Imported Into Japan?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Resilient Infrastructure Ppps 15 1.3 Scope and Objectives of This Study 19 1.4 Selection of Cases for the Japan Case Study 20 1.5 Structure of This Report 21
Resilient Infrastructure Public-Private Partnerships (PPPs): Contracts and Procurement Contracts Public-Private Infrastructure Partnerships (PPPs): Resilient Resilient Infrastructure Public Disclosure Authorized Public-Private Partnerships (PPPs): Contracts and Procurement Public Disclosure Authorized The Case of Japan The Case of Japan The Case Public Disclosure Authorized Public Disclosure Authorized ©2017 The World Bank International Bank for Reconstruction and Development The World Bank Group 1818 H Street NW, Washington, DC 20433 USA December 2017 DISCLAIMER This work is a product of the staff of The World Bank with external contributions. The findings, interpretations, and conclusions expressed in this work do not necessarily reflect the views of The World Bank, its Board of Executive Directors, or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. Nothing herein shall constitute or be considered to be a limitation upon or waiver of the privileges and immunities of The World Bank, all of which are specifically reserved. The report reflects information available up to November 30, 2017. RIGHTS AND PERMISSIONS The material in this work is subject to copyright. Because The World Bank encourages dissemination of its knowledge, this work may be reproduced, in whole or in part, for noncommercial purposes as long as full attribution to this work is given. Any queries on rights and licenses, including subsidiary rights, should be addressed to World Bank Publications, The World Bank Group, 1818 H Street NW, Washington, DC 20433, USA; e-mail: [email protected]. -

Relationship with Society
Relationship with Society Basic Concept ② Contribute to Society and the Environment Under the Group Action Guideline to “Contribute to society,” the Forestation Project ANA Group has been actively working on environmental and social Since 2004, the ANA issues as a good corporate citizen. Taking into account various factors Group has been involved including the ANA Group’s Corporate Philosophy, business activities in a ten-year forestation and management resources, the Group set forth three activity project in areas domains it should undertake: 1) support people through air travel, surrounding the approxi- 2) contribute to society and the environment, and 3) contribute to the mately 50 domestic community. In pursuing social contribution activities unique to ANA, airports it serves. In the the ANA Group attaches importance to active involvement of employ- fiscal year ended March ees in volunteer activities and creation of opportunities for joint, Forest thinning at Echigo Heiya Forest near 2011, employees and Niigata Airport empathetic activities with its customers. local volunteers worked together at four new airports in Nemuro-Nakashibetsu, Niigata, Komatsu and Hagi-Iwami, for forestation activities at a total of ① Support people ② Contribute to society ten locations. through air travel and the environment ◆ History of Forestation Activities ③ Contribute to the community (Fiscal Year Ended March 2011) Name Nearest Airport Frequency Details Shimafukuro Forest Nemuro-Nakashibetsu First time Afforestation The ANA Character Rinkoshi Forest -

Shikoku Access Map Matsuyama City & Tobe Town Area
Yoshikawa Interchange Hiroshima Airport Okayama Airport Okayama Kobe Suita Sanyo Expressway Kurashiki Junction Interchange Miki Junction Junction Junction Shikoku Himeji Tarumi Junction Itami Airport Hiroshima Nishiseto-Onomichi Sanyo Shinkansen Okayama Hinase Port Shin-Kobe Shin- Okayama Interchange Himeji Port Osaka Hiroshima Port Kure Port Port Obe Kobe Shinko Pier Uno Port Shodoshima Kaido Shimanami Port Tonosho Rural Experience Content Access Let's go Seto Ohashi Fukuda Port all the way for Port an exclusive (the Great Seto Bridge) Kusakabe Port Akashi Taka Ikeda Port experience! matsu Ohashi Shikoku, the journey with in. Port Sakate Port Matsubara Takamatsu Map Tadotsu Junction Imabari Kagawa Sakaide Takamatsu Prefecture Kansai International Imabari Junction Chuo Airport Matsuyama Sightseeing Port Iyosaijyo Interchange Interchange Niihama Awajishima Beppu Beppu Port Matsuyama Takamatsu Airport 11 11 Matsuyama Kawanoe Junction Saganoseki Port Tokushima Wakayama Oita Airport Matsuyama Iyo Komatsu Kawanoe Higashi Prefecture Naruto Interchange Misaki Interchange Junction Ikawa Ikeda Interchange Usuki Yawata Junction Wakimachi Wakayama Usuki Port Interchange hama Interchange Naruto Port Port Ozu Interchange Ehime Tokushima Prefecture Awa-Ikeda Tokushima Airport Saiki Yawatahama Port 33 32 Tokushima Port Saiki Port Uwajima Kochi 195 Interchange Hiwasa What Fun! Tsushima Iwamatsu Kubokawa Kochi Gomen Interchange Kochi Prefecture 56 Wakai Kanoura ■Legend Kochi Ryoma Shimantocho-Chuo 55 Airport Sukumo Interchange JR lines Sukumo Port Nakamura -
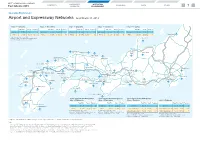
7. Airport and Expressway Networks (PDF, 352KB)
WEST JAPAN RAILWAY COMPANY CORPORATE OPERATING CONTENTS BUSINESS DATA OTHER Fact Sheets 2019 OVERVIEW ENVIRONMENT 7 Operating Environment Airport and Expressway Networks As of March 31, 2019 Tokyo — Fukuoka Tokyo — Hiroshima Tokyo — Okayama Tokyo — Kanazawa Tokyo — Toyama Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Shinkansen 4h 46m 22,950 31 Shinkansen 3h 44m 19,080 46 Shinkansen 3h 09m 17,340 60 Shinkansen 2h 28m 14,120 24 Shinkansen 2h 08m 12,730 24 Niigata Airport Airlines 3h 00m 41,390 54 (19) Airlines 3h 30m 34,890 18 Airlines 3h 10m 33,990 10 Airlines 2h 50m 24,890 10 Airlines 2h 30m 24,890 4 Travel Time and Fare: JAL or ANA Noto Airport Frequency: All airlines. Numbers in parentheses are frequency excluding those of JAL or ANA. Kanazawa Izumo Airport Komatsu Toyama Airport Yonago Airport Airport Tottori Airport Yonago Hagi Iwami Airport Izumo Tajima Airport Gotsu Hamada Tsuruga Yamaguchi Ube Airport Yamaguchi HiroshimaHiroshima Hiroshima Airport Okayama Airport Maibara Kitakyushu Ibaraki Airport Onomichi Hakata KomakiKomaki AirportAirport Okayama KobeKobe ItamiItami AirportAirport Fukuoka Airport Kitakyushu Airport KKurashikiurashiki SSuitauita Iwakuni Kintaikyo NagoyaNagoya Sasebo Tosu Airport Sakaide Shin-OsakaShin-Osaka Tokyo Saga Airport Imabari Kobe Airport Narita Airport Matsuyama Airport Takamatsu Airport Naruto KansaiKansai AirportAirport Haneda Airport Oita Airport Kansai Nagasaki International Airport Chubu International -

Analysis of the Effects of Air Transport Liberalisation on the Domestic Market in Japan
Chikage Miyoshi Analysis Of The Effects Of Air Transport Liberalisation On The Domestic Market In Japan COLLEGE OF AERONAUTICS PhD Thesis COLLEGE OF AERONAUTICS PhD Thesis Academic year 2006-2007 Chikage Miyoshi Analysis of the effects of air transport liberalisation on the domestic market in Japan Supervisor: Dr. G. Williams May 2007 This thesis is submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy © Cranfield University 2007. All rights reserved. No part of this publication may be reproduced without the written permission of the copyright owner Abstract This study aims to demonstrate the different experiences in the Japanese domestic air transport market compared to those of the intra-EU market as a result of liberalisation along with the Slot allocations from 1997 to 2005 at Haneda (Tokyo international) airport and to identify the constraints for air transport liberalisation in Japan. The main contribution of this study is the identification of the structure of deregulated air transport market during the process of liberalisation using qualitative and quantitative techniques and the provision of an analytical approach to explain the constraints for liberalisation. Moreover, this research is considered original because the results of air transport liberalisation in Japan are verified and confirmed by Structural Equation Modelling, demonstrating the importance of each factor which affects the market. The Tokyo domestic routes were investigated as a major market in Japan in order to analyse the effects of liberalisation of air transport. The Tokyo routes market has seven prominent characteristics as follows: (1) high volume of demand, (2) influence of slots, (3) different features of each market category, (4) relatively low load factors, (5) significant market seasonality, (6) competition with high speed rail, and (7) high fares in the market. -

Wisconsin Veterans Museum Research Center Transcript of an Oral
Wisconsin Veterans Museum Research Center Transcript of an Oral History Interview with JOEL C. MICKELSON Radar Repairman, Army Air Corps, World War II. 2000 OH 299 1 OH 299 Mickelson, Joel C., (1925-2011). Oral History Interview, 2000. User Copy: 1 sound cassette (ca. 57 min.), analog, 1 7/8 ips, mono. Master Copy: 1 sound cassette (ca. 57 min.), analog, 1 7/8 ips, mono. Video Recording: 1 videorecording (ca. 57 min.); ½ inch, color. Transcript: 0.1 linear ft. (1 folder). Abstract: Joel Mickelson, a Willmar, Minnesota native, discusses his World War II service with the Army Air Corps as a radar repairman in the Pacific theater of operations. Mickelson mentions being drafted and sent to basic training at Lincoln Air Base (Nebraska). He talks about radio school in South Dakota, reassignment to repair school at Truax Field (Wisconsin), and electronics training at Chanute Field (Illinois). After a furlough at home, he portrays the ship ride from California to Leyte (Philippines). Mickelson describes living in tents during the rainy season, taking Atabrine to prevent malaria, and developing tinnitus. He talks about maintaining aircraft radar equipment and being moved from Luzon to Lingayen Air Base to Clark Air Base. Mickelson touches on liberty in Manila and occasional visits from the Red Cross. While he was off duty, he tells of exploring a cave full of Japanese equipment and shooting a huge lizard. Transferred to Naha (Okinawa), he comments on working in the 39 th Squadron, 35 th Fighter Group office and being promoted to assistant for the director of communications. -

LUGGAGE-FREE TRAVEL Same-Day Delivery
LUGGAGE-FREE TRAVEL Same-day Delivery ◆Airport ✈ → Hotel Delivery Delivery City Drop-off Earliest delivery From To Narita Airport 6:30ー10:00 Tokyo(Chiba) Ibaraki、Tochigi、Gunma、Saitama、Chiba、Tokyo、Kanagawa、Yamanashi Haneda airport 00:00ー10:30 Osaka Kansai International Airport 6:30ー9:30 Osaka、Kyoto、Hyogo、Nara、Shiga Chubu International Airport Terminal 1 7:00ー11:00 Aichi、Mie、Gifu Nagoya 18:00ー21:00 Chubu International Airport Terminal 2 10:00ー11:00 Aichi、Mie、Gifu Sendai Station Sendai International Airport 8:00ー9:30 Within Miyagi Sapporo New Chitose Airport 7:30ー10:00 Within Sapporo Cuty Fukuoka Fukuoka Airport 7:30ー11:30 Within Chuo Area, Fukuoka Area *Luggage that is dropped-off after the above mentioned time, but before 18:00 will be delivered by the next day. ◆Station → Hotel Delivery Delivery City・Station Drop-off Earliest delivery From To Yamato Transport Asahikawa Station Kitasaito Center (JR Within Asahikawa Area・Furano City・Within Sounkyo-Onsen Kamikawagun Asahikawa Station 8:00ー16:30 Asahikawa Station) Kamikawa Town Yamato Transport Sendai Station 2F Baggage Service Sendai Station 9:00ー10:30 Within Miyagi Counter (JR Sendai Station) Ginza Yamato Transport Ginza Konyabashi Center (Tokyo Metro 8:00ー11:00 Ibaraki、Tochigi、Gunma、Saitama、Chiba、Tokyo、Kanagawa、Yamanashi Yurakucho Staion Ginza Station, JR Yurakucho Station) Yokohama Yamato Transport Sakuragicho Station Tourist Information Sakuragcho 9:00ー10:00 Ibaraki、Tochigi、Gunma、Saitama、Chiba、Tokyo、Kanagawa、Yamanashi Center (JR Sakuragicho Station) Station Nagano Yamato Transport MIDORI -

Kirin Holdings Co. Ltd in Beer - World
Kirin Holdings Co. Ltd in Beer - World June 2010 Scope of the Report Kirin Holdings - Beer © Euromonitor International Scope • 2009 figures are based on part-year estimates. • All forecast data are expressed in constant terms; inflationary effects are discounted. Conversely, all historical data are expressed in current terms; inflationary effects are taken into account. • Alcoholic Drinks coverage: Alcoholic Drinks 235 billion litres RTDs/ Wine Beer Spirits High-strength Cider/perry 27 bn litres 184 bn litres 19 bn litres Premixes 1.5 bn litres 4 bn litres Note: Figures may not add up due to rounding Disclaimer Learn More Much of the information in this briefing is of a statistical To find out more about Euromonitor International's complete nature and, while every attempt has been made to ensure range of business intelligence on industries, countries and accuracy and reliability, Euromonitor International cannot be consumers please visit www.euromonitor.com or contact your held responsible for omissions or errors local Euromonitor International office: Figures in tables and analyses are calculated from London + 44 (0)20 7251 8024 Vilnius +370 5 243 1577 unrounded data and may not sum. Analyses found in the Chicago +1 312 922 1115 Dubai +971 4 372 4363 briefings may not totally reflect the companies’ opinions, Singapore +65 6429 0590 Cape Town +27 21 552 0037 reader discretion is advised Shanghai +86 21 63726288 Santiago +56 2 915 7200 2 Kirin Holdings - Beer © Euromonitor International Strategic Evaluation Competitive Positioning Market Assessment Category and Geographic Opportunities Operations Brand Strategy Recommendations 3 Strategic Evaluation Kirin Holdings - Beer © Euromonitor International Kirin Company Facts Kirin Kirin has looked for overseas expansion Headquarters Tokyo, Japan • Kirin has expanded its presence internationally, particularly in Asia Pacific and Australasia. -
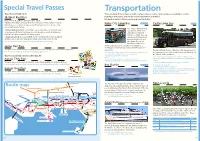
Transportation Travel Economically on JR There Are Many Different Types of Public Transportation, As Well As Tickets and Passes Available to Tourists
Special Travel Passes Transportation Travel Economically on JR There are many different types of public transportation, as well as tickets and passes available to tourists. JR-West Rail Pass By using tourist passes, you can also receive discounts on your travel. Information centers offer you route maps and timetables. Passes for short-term foreign travelers to Japan. •[Kansai Area Pass] can be used on main railways in Kobe, Osaka, and Kyoto. Kobe City Loop Bus MAP P33,P34 Castle Loop Bus MAP P35 Unlimited access to Limited express train Haruka (non-reserved seats only) excluding the bullet trains. The City Loop Bus will take • [Kansai Wide Area Pass] is providing 5 consecutive days of unlimited travel you around all the main on designated JR trains (including unreserved seats on selected shinkansen attractions. It takes 63 and Limited express trains) in the Kansai region. minutes to make one loop • [Sanyo Area Pass] gives you unlimited ride on JR trains from Kansai Airport, and connects 16 bus stops and between Osaka and Fukuoka including Sanyo Bullet Train Nozomi. between Kobe Port and For more information : http://www.jr-odekake.net/en/jwrp/ the Kitano Foreigners Residences. Videos are Japan Rail Pass shown at the bus about each site. If you purchase a one-day pass, it will get you a discount at many tourist This pass offers unlimited travel on any JR train in the country. (There are some exceptions.) attractions. This bus will take you on a ride to see the famous garden For more information : http://www.japanrailpass.net/ A one day ticket comes with coupons for various tourist attractions. -

Flight Path to New Horizons Annual Report 2012 for the Year Ended March 31, 2012
Flight Path to New Horizons Annual Report 2012 For the Year Ended March 31, 2012 Web Edition Shinichiro Ito President and Chief Executive Officer Editorial Policy The ANA Group aims to establish security and reliability through communication with its stakeholders, thus increasing corporate value. Annual Report 2012 covers management strategies, a business overview and our management struc- ture, along with a wide-ranging overview of the ANA Group’s corporate social responsibility (CSR) activities. We have published information on CSR activities that we have selected as being of particular importance to the ANA Group and society in general. Please see our website for more details. ANA’s CSR Website: http://www.ana.co.jp/eng/aboutana/corporate/csr/ Welcome aboard Annual Report 2012 The ANA Group targets growth with a global business perspective. Based on our desire to deliver ANA value to customers worldwide, our corporate vision is to be one of the leading corporate groups in Asia, providing passenger and cargo transportation around the world. The ANA Group will achieve this vision by responding quickly to its rapidly changing operating environment and continuing to innovate in each of its businesses. We are working toward our renaissance as a stronger ANA Group in order to make further meaningful progress. Annual Report 2012 follows the ANA Group on its journey through the skies as it vigorously takes on new challenges to get on track for further growth. Annual Report Flight 2012 is now departing. Enjoy your flight! Targeted Form of the ANA Group ANA Group Corporate Philosophy ANA Group Corporate Vision Our Commitments On a foundation of security and reliability, With passenger and cargo the ANA Group will transportation around the world • Create attractive surroundings for customers as its core field of business, • Continue to be a familiar presence the ANA Group aims to be one of the • Offer dreams and experiences to people leading corporate groups in Asia. -

TIME TABLE Kansai International Airport - Osaka (Itami) Airport
From Kansai International Airport to Koriyama, and Vice Versa JAPAN The Sea of Japan Koriyama Railway Station Osaka (Itami) Airport Fukushima Airport Tsukuba Science City Tokyo Narita International Airport Kansai International Airport The Pacific Ocean There is no direct way to get Koriyama from Kansai International Airport. The earlier and easier way is as follows: First, you have to go to Osaka (Itami) Airport by limousine bus from Kansai International Airport. It takes about 75 minutes and the bus fare is 1,700 yen. Next, you go to Fukushima Airport by domestic air from Osaka (Itami) Airport. It takes about 65 minutes and the air fare is 25,500 - 27,500 yen. Finally, you go to Koriyama Railway station by limousine bus from Fukushima Airport. It takes about 40 minutes and the bus fare is 800 yen. You can also take a taxi from Fukushima Airport to Koriyama Railway station, it takes about 30 minutes and the taxi fare is about 6,000 yen. All the hotels for ECM participants are located near Koriyama Railway Station, that is, in downtown Koriyama. 1 Kansai International Airport Guide To Koriyama Terminal building From foreign countries, arrival lobby is located at 4F the first floor of the terminal building. To Osaka Departure lobby for International Airlines (Itami) Airport, you can buy a bus ticket at the ticket counter on the first floor and take the bus at 3F Bus Stop No.8. Restaurant and From Koriyama 2F From Osaka (Itami) Airport, the bus arrive at the Lobby for Domestic Airlines forth floor. To foreign countries, departure lobby is 1F located at the forth floor of the terminal building. -

Activities in Japan 1 Activities in Japan
Chapter 3 Activities in Japan 1 Activities in Japan (1) Schedule Date Time Program October 27 <National Leaders (NLs), Participating Youths (PYs) and host family representatives Tuesday from ASEAN member countries> Arrival at Narita International Airport 6:45 Myanmar (NH-814) 7:15 Malaysia, Brunei Darussalam (MH-088) 7:35 Lao P.D.R., Cambodia (TG-642) 8:00 Host family representatives from Vietnam (VN-300) 8:50 Indonesia (GA-874) *arrival at Haneda airport Transfer to the Cabinet Office for orientation Move to Hotel New Otani Tokyo 15:00 Philippines (NH-820) 15:05 Vietnam (VN-384) *arrival at Haneda airport 16:05 Singapore (JL-712) 17:30 Thailand (JL-032)*arrival at Haneda airport Transfer to Hotel New Otani Tokyo and orientation at the hotel Stay at Hotel New Otani Tokyo <Japanese PYs> Pre-departure training Stay at National Olympics Memorial Youth Center October 28 <Japanese PYs> Wednesday 8:15 Move to Hotel New Otani Tokyo <NLs, PYs and host family representatives> 9:00-11:00 Orientation (“Ho-oh”, Hotel New Otani Tokyo) • Speech by Mr. Hideki Uemura, Administrator • Introduction of NLs and PYs • Introduction of host family representatives • Introduction of Administrative staff members • Explanation of the country program in Japan • Speech by Ms. Tomoko Okawara, Chairperson of Japan-ASEAN Youth Leaders Summit (YLS) Organizing Committee • Solidarity Group (SG) meeting <Host family representatives> 11:15-11:45 Courtesy call on Mr. Takahiko Yasuda, Director General for International Youth Exchange, Cabinet Office (“Tsubaki”, Hotel New Otani Tokyo) • Speech by Mr. Takahiko Yasuda, Director General for International Youth Exchange, Cabinet Office • Presentation of certificate and gift • Photo session 30 Chapter 3 Activities in Japan Date Time Program October 28 <NLs, PYs and host family representatives> Wednesday 12:00-12:30 Inauguration Ceremony (“Ho-oh”, Hotel New Otani Tokyo) • Moment of silence for the victims of the bus accident in Brunei Darussalam in 2001 • Speech by Mr.