Report 2010–5070–F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Françoisite-(Ce)
American Mineralogist, Volume 95, pages 1527–1532, 2010 Françoisite-(Ce), a new mineral species from La Creusaz uranium deposit (Valais, Switzerland) and from Radium Ridge (Flinders Ranges, South Australia): Description and genesis NICOLAS MEISSER,1,* JOËL BRUGGER,2,3 STEFA N AN SER M ET ,1 PHILI pp E THÉLI N ,4 A N D FRA N ÇOIS BUSSY 5 1Musée de Géologie and Laboratoire des Rayons-X, Institut de Minéralogie et de Géochimie, UNIL, Anthropole, CH-1015 Lausanne-Dorigny, Switzerland 2South Australian Museum, North Terrace, 5000 Adelaide, Australia 3TRaX, School of Earth and Environmental Sciences, University of Adelaide, 5005 Adelaide, Australia 4Laboratoire des Rayons-X, Institut de Minéralogie et de Géochimie, UNIL, Anthropole, CH-1015 Lausanne-Dorigny, Switzerland 5Laboratoire de la microsonde électronique, Institut de Minéralogie et de Géochimie, UNIL, Anthropole, CH-1015 Lausanne-Dorigny, Switzerland AB STRACT The new mineral françoisite-(Ce), (Ce,Nd,Ca)[(UO2)3O(OH)(PO4)2]·6H2O is the Ce-analog of françoisite-(Nd). It has been discovered simultaneously at the La Creusaz uranium deposit near Les Marécottes in Valais, Switzerland, and at the Number 2 uranium Workings, Radium Ridge near Mt. Painter, Arkaroola area, Northern Flinders Ranges in South Australia. Françoisite-(Ce) is a uranyl- bearing supergene mineral that results from the alteration under oxidative conditions of REE- and U4+- bearing hypogene minerals: allanite-(Ce), monazite-(Ce), ±uraninite at Les Marécottes; monazite-(Ce), ishikawaite-samarskite, and an unknown primary U-mineral at Radium Ridge. The REE composition of françoisite-(Ce) results from a short aqueous transport of REE leached out of primary minerals [most likely monazite-(Ce) at Radium Ridge and allanite-(Ce) at La Creusaz], with fractionation among REE resulting mainly from aqueous transport, with only limited Ce loss due to oxidation to Ce4+ during transport. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Heat Capacities and Thermodynamic Functions for Beryl, Beralrsiuotr
American Mineralogist, Volume 7I, pages 557-568, 1986 Heat capacitiesand thermodynamicfunctions for beryl, BerAlrSiuOtr, phenakite, BerSiOn,euclase, BeAlSiOo(OH)' bertranditeo BeoSirOt(OH)r,and chrysoberyl' BeAl2Oa B. S. HnluNcw.q,Y U.S. GeologicalSurvey, Reston, Y irgrnia22092 M. D. B.nnroN Departmentof Earthand SpaceSciences, University of California,Los Angeles,Los Angeles,California 90024 R. A. Ronrn, H. T. HLsnr.roN, Jn. U.S. GeologicalSurvey, Reston, Y irginia22092 Ansrru,cr The heat capacities of beryl, phenakite, euclase,and bertrandite have been measured betweenabout 5 and 800 K by combined quasi-adiabaticcryogenic calorimetry and dif- ferential scanningcalorimetry. The heat capacitiesof chrysoberylhave beenmeasured from 340 to 800 K. The resulting data have been combined with solution and phase-equilibrium experimentaldata and simultaneouslyfit using the program pHAS2oto provide an internally consistent set of thermodynamic properties for several important beryllium phases.The experimentalheat capacitiesand tablesof derived thermodynamic propertiesare presented in this report. The derived thermodynamic properties at I bar and 298.15 K for the stoichiometric beryllium phasesberyl, phenakite, euclase,and bertrandite are entropies of 346.7 + 4.7, 63.37+0.27,89.09+0.40, andl72.l+0.77 J/(mol'K),respectively,andGibbsfree energiesof formation(elements) of -8500.36 + 6.39, -2028.39 + 3.78, -2370.17 + 3.04, and -4300.62 + 5.45 kJlmol, respectively,and, -2176.16 + 3.18kJ/mol for chrysoberyl. The coefficientscr to c, of the heat-capacityfunctions are as follows: valid phase ct c2 c, x 105 c4 c, x 10-6 range beryl 1625.842 -0.425206 12.0318 -20 180.94 6.82544 200-1800K phenakite 428.492 -0.099 582 1.9886 -5 670.47 2.0826 200-1800K euclase 532.920 -0.150729 4.1223 -6726.30 2.1976 200-1800K bertrandite 825.336 -0.099 651 -10 570.31 3.662r7200-1400 K chrysoberyl 362.701 -0.083 527 2.2482 -4033.69 -6.7976 200-1800K whereC!: cr * ctT + crT2 * coT-os* crT-2and Zisinkelvins. -

Weeksite K2(UO2)2(Si5o13)·4H2O
Weeksite K2(UO2)2(Si5O13)·4H2O Crystal Data: Monoclinic. Point Group: 2/m: As bladed or acicular crystals, flattened on {010} and elongated along [001], and as flat plates; also as spherulites and radiating fibrous clusters. - Twinning: By two-fold rotation around [401 ]. Physical Properties: Cleavage: Two good prismatic cleavages noted. Hardness = < 2 D(meas.) = ~ 4.1 D(calc.) = 3.80 Radioactive. Optical Properties: Transparent to translucent. Color: Yellow. Luster: Waxy to silky. Optical Class: Biaxial (-). α = 1.596 β = 1.603 γ = 1.606 2V(meas.) = ~ 60° 2V(calc.) = 66° Pleochroism: X = colorless, Y = pale yellow-green, Z = yellow-green. Dispersion: r > v, strong. Orientation: X = b, Y = c, Z = a. Cell Data: Space Group: C2/m. a = 14.26(2) b = 35.88(10) c = 14.20(2) β = 111.578(3)° Z = 4 X-ray Powder Pattern: Thomas Range, Utah, USA. 7.11 (10), 5.57 (9), 8.98 (8), 3.55 (7), 3.30 (7), 2.91 (6), 3.20 (5) Chemistry: (1) (1) Na2O 0.53 Al2O3 0.6 K2O 4.73 SiO2 29.44 CaO 0.67 UO3 55.78 BaO 3.11 H2O [7.02] MgO 0.18 Total 101.28 SrO 0.20 (1) Anderson mine, Arizona, USA; average of 8 electron microprobe analyses, supplemented by TGA, H2O calculated from structure, corresponds to (K1.031Na0.176Ca0.123Ba0.208)Σ=1.537(UO2)2.002 (Si5.030O13)·4H2O. Occurrence: In “opal" veinlets in rhyolite, agglomerates, sandstones and limestones. Association: “Opal," “chalcedony," calcite, gypsum, fluorite, uraninite, thorogummite, uranophane, boltwoodite, carnotite, margaritasite. Distribution: In the USA, in Utah, at the Autunite No. -

Abstract Spectroscopic Characterization of Fluorite
ABSTRACT SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR By Carrie Wright This thesis consists of two separate papers on color in fluorite. In the first paper, synthetic fluorites doped with various REEs (10-300 ppm) were analyzed using direct current plasma spectrometry, optical absorption spectroscopy, fluorescence spectrophotometry, and electron paramagnetic resonance spectroscopy before and after receiving 10-25 Mrad of 60Co gamma irradiation. The combined results of these techniques indicate that the irradiation-induced color of the Y-, Gd-, La- and Ce-doped samples are the result of a REE-associated fluorine vacancy that traps two electrons. Divalent samarium may be the cause of the irradiation-induced green color of the Sm- doped sample. In the second paper, fluorite crystals from Bingham, NM, Long Lake, NY, and Westmoreland, NH were similarly investigated to determine the relationship between sectorally zoned trace elements, defects, and color. The results indicate causes of color similar to those in the synthetic samples with the addition of simple F-centers. SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR A Thesis Submitted to the Faculty of Miami University In partial fulfillment of The requirements for the degree of Master of Science Department of Geology By Carrie Wright Miami University Oxford, OH 2002 Advisor_____________________ Dr. John Rakovan Reader______________________ Dr. Hailiang Dong TABLE OF CONTENTS Chapter 1: Introduction to the cause of color in fluorite 1 Manuscript 1-Chapter 2 29 “Spectroscopic investigation of lanthanide doped CaF2 crystals: implications for the cause of color” Manuscript 2-Chapter 3 95 “Spectroscopic characterization of fluorite from Bingham, NM, Long Lake, NY and Westmoreland, NH: relationships between trace element zoning, defects and color ii TABLE OF FIGURES Chapter 1 Figures 21 Figure 1a. -

Minerals of the Eudialyte Group from the Sagasen Larvikite Quarry, Porsgrunn, Norway
= Minerals of the eudialyte group from the Sagasen larvikite quarry, Porsgrunn, Norway Alf Olav Larsen, Arne Asheim and Robert A. Gault Introduction Eudialyte, aNa-rich zirconosilicate with varying amounts of Ca, Fe, Mn, REE, Nb, K, Y, Ti, CI and F, was first described from the llimaussaq alkaline complex, South Greenland by Stromeyer (1819), Since then, the mineral has been described from many other alkaline deposits, and is a characteristic mineral in agpaitic nepheline syenites and their associated pegmatites. In recent years, eudialyte (sensa lata) has been the subject of extensive studies. The broad compositional variations and new insight into the crystal chemistry of the mineral group resulted in the definition of several new species by the Eudialyte Nomenclature Subcommittee under the Commission on New Minerals and Mineral Names of the International Mineralogical Association (Johnsen et al. 2003b). Brown eudialyte (s. I.) is a common constituent of the agpaitic pegmatites in the Langesundsfjord district in the western part of the Larvik plutonic complex (Br0gger 1890). Recent chemical analyses of the mineral have shown that some localities contain ferrokentbrooksite (Johnsen et al. 2003a). Other localities hold eudialyte (sensa stricto). Ferrokentbrooksite is the ferrous-iron-dominant analogue of kentbrooksite with Fe as the predominant element replacing Mn. Kentbrooksite is the Mn-REE-Nb-F end member in a solid solution series between eudialyte (s. s.) and ferrokentbrooksite, with an extension to oneillite (Johnsen et al. 1998, Johnsen et al. 1999, Johnsen et al. 2003a), as well as to carbokentbrooksite and zirsilite-(Ce) (Khomyakov et al. 2003). Carbokentbrooksite has a significant content of carbonate and Na > REE for the N4 site, while zirsilite-(Ce) has REE > Na (with Ce predominant) for the N4 site. -

Predicting Berborite As Potential Deep-Ultraviolet Nonlinear Optical Crystal from First Principles
Predicting Berborite as Potential Deep-Ultraviolet Nonlinear Optical Crystal from First Principles Lei Kang,1,2 Pifu Gong,1 Zheshuai Lin,1* Bing Huang2† 1 Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China 2 Beijing Computational Science Research Center, Beijing 100193, China E-mails: [email protected] (Z.L.); [email protected] (B.H.) Following our ab initio nonlinear optical (NLO) materials design guidelines, in this Letter, we discovered a novel type of structure to realize potential deep-ultraviolet (DUV) NLO performance in the classical beryllium borate system. By densely stacking the NLO-active layered frameworks, the key design scheme for the structural evolution from the (Be2BO3F2)∞ layers in KBe2BO3F2 (KBBF) to the novel (Be2BO5H3)∞ layers in berborite is illustrated. Based on available experimental results and systematical theoretical evaluation from first principles, the NLO properties of berborite are further obtained as comparable as the only pratical DUV NLO crystal KBBF. It is demonstrated that berborite can achieve available DUV phase-matched output with strong NLO effect for the practically important 177.3 nm and 193.7 nm lasers. Once obtained with sizable single crystal, it can be applied as a promising DUV NLO crystal. Deep-ultraviolet (DUV, λ ≤ 200 nm) nonlinear optical (NLO) crystals are important optoelectronic materials for providing scientists with powerful harmonic laser sources for industrial production (e.g., 193.7 nm lithography) and fundamental research (e.g., 177.3 nm photoelectron spectroscopy) [1-9]. However, DUV NLO crystals are rare because it is extremely challenging to achieve phase-matching (PM) condition in the DUV region [10-12]. -

Contributions to the Mineralogy of Norway
NORSK GEOLOGISK TIDSSKRIFT 45 CONTRIBUTIONS TO THE MINERALOGY OF NORWAY No. 30. Minerals from Nordmarkite Druses BY IVAR OFTEDAL and P. CHR. SÆBO (Institutt for geologi, Blindern, Oslo 3) Abstract. Crystals of phenacite, milarite, bertrandite, ancylite, wulfenite, pyrosmalite, and harmotome occur in nordmarkite druses in the Grorud district. The Grorud milarite contains substantial amounts of yttrium and yttrium earths. Milarite and pyrosmalite are new species for Norway. It is well known that the boundary zone of the nordmarkite in the Grorud district, near Oslo, is rich in druses and that several rarer min erals have been found in them, e.g. sphene, zircon, allanite, helvine. Recently, additional species have been found in a quarry at Flaen in eastern Grorud. The nordmarkite here is dissected by aplitic veins and unusually rich in druses and o pen fissures containing a fair amount of rare minerals, some of them previously mentioned (OFTEDAL and SÆBO 1963). We intend to examine more closely some of the minerals mentioned below. The principal purpose of the present note is to announce new finds. Phenacite is a new species in the Permian rocks of the Oslo Region. It occurs sparsely as transparent and colourless crystals, mostly smal ler than l mm. By careful inspection of a number of druses we now have 12 crystals altogether, all but one grown on faces of orthoclase, the one sits on a quartz rhombohedron face. The phenacite crystals are partly enclosed by orthoclase. We did not try to loosen them and thus actual measurements of face angles were not carried out. However, it can be seen that they are bounded by a vertically striated prism zone, which is usually very short, and a predominant rather flat rhombo hedron, probably {1011}, which is strongly etched (Fig. -
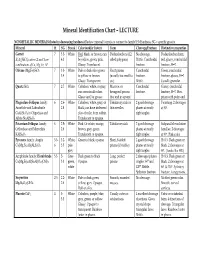
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Geology of the Pegmatites and Associated Rocks of Maine
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR BULLETIN 445 GEOLOGY OF THE PEGMATITES AND ASSOCIATED ROCKS OF MAINE INCLUDING FELDSPAR, QUARTZ, MICA, AND GEM DEPOSITS BY EDSON S. BASTIN WASHINGTON GOVERNMENT PRINTING OFFICE 1911 CONTENTS. Introduction.............................................................. 9 Definition of pegmatite...................................................... 10 Geographic distribution.................................................... 10 Geology.................................................................. 10 Bordering rocks....................................................... 10 Pegmatites in foliated rocks........................................ 11 General statement............................................ 11 Sedimentary foliates........................................... 11 Igneous foliates.....".......................................... 12 Pegmatites in massive granites.................................... 13 'Age.................................................................. 15 General character..................................................... 15 Mineral and chemical composition................................. 15 Mineral constituents.......................................... 15 Relative proportions of minerals............................... 18 Quartzose phases. ..............................^............. 18 Fluidal cavities............................................... 19 Sodium and lithium phases................................... 20 Muscovite -

Download the Scanned
American Mineralogist, Volume 63, pages 664_676, l97g Multisyste_msanalysis of beryiliumminerar stabilities: the systemBeO-A[rOa_SiO2_H2O DoNer.n M. Bunr Department of Geology, Arizona State (Jniuersitv Tempe,Arizona8528I Abstract seven commonly associatedminerals in the systemBeo-Alror-Sior-Hro includechry- soberyl,phenakite, euclase,bertrandite, beryl, kaolinite,and qiuit . The phaserule implies that not more than six of theseminerals can coexistat an invariantpoint, and, with the addition of an aqueousphase, the associationconstitutes an (n * 4; ptrase(negative two d-egreesof freedom)multisystem. The apparentincompatibility of taotinite with phenakite allowsthe splitting of this unwieldymultisystem into two smaller(n + 3) phasemultisystems, which may belabeled (Kao) and (phe).Moiar volumedata, compuier program RrlcrroN, and naturalassemblages can then be usedto derivethe presumablystabie cJnfiguration of these multisystemson p"-minus an isothermal pHro diagram. n, p-r diagramprojected through theaqueous phase shouldhave the sametopology, and cansimilarlf be drawn. on the resultingdiagrams, three . invariant-butpoinis rabeled tchrl, tBr;;, and [etz] arestable in the.multisystem (Kao), and threedistinct identically-fuU"i.Opoint, u.. stablein rhe multisystem(Phe). An implicationof this topologyvia ihe "r.tu.tubl"-rtable correspon- dence,"is that the assemblagephenakite + euclase* beryl(* aqueousphase) has a finite i'I;ix, ii,l'J.?lT"t' ::ff,,,j :r;: :":.T' ? ts why" euclase is muchrarer than bertrandite. and its stabilityfield, especially -

Winter 1998 Gems & Gemology
WINTER 1998 VOLUME 34 NO. 4 TABLE OF CONTENTS 243 LETTERS FEATURE ARTICLES 246 Characterizing Natural-Color Type IIb Blue Diamonds John M. King, Thomas M. Moses, James E. Shigley, Christopher M. Welbourn, Simon C. Lawson, and Martin Cooper pg. 247 270 Fingerprinting of Two Diamonds Cut from the Same Rough Ichiro Sunagawa, Toshikazu Yasuda, and Hideaki Fukushima NOTES AND NEW TECHNIQUES 281 Barite Inclusions in Fluorite John I. Koivula and Shane Elen pg. 271 REGULAR FEATURES 284 Gem Trade Lab Notes 290 Gem News 303 Book Reviews 306 Gemological Abstracts 314 1998 Index pg. 281 pg. 298 ABOUT THE COVER: Blue diamonds are among the rarest and most highly valued of gemstones. The lead article in this issue examines the history, sources, and gemological characteristics of these diamonds, as well as their distinctive color appearance. Rela- tionships between their color, clarity, and other properties were derived from hundreds of samples—including such famous blue diamonds as the Hope and the Blue Heart (or Unzue Blue)—that were studied at the GIA Gem Trade Laboratory over the past several years. The diamonds shown here range from 0.69 to 2.03 ct. Photo © Harold & Erica Van Pelt––Photographers, Los Angeles, California. Color separations for Gems & Gemology are by Pacific Color, Carlsbad, California. Printing is by Fry Communications, Inc., Mechanicsburg, Pennsylvania. © 1998 Gemological Institute of America All rights reserved. ISSN 0016-626X GIA “Cut” Report Flawed? The long-awaited GIA report on the ray-tracing analysis of round brilliant diamonds appeared in the Fall 1998 Gems & Gemology (“Modeling the Appearance of the Round Brilliant Cut Diamond: An Analysis of Brilliance,” by T.