A Brief Overview Including Uses, Worldwide Resources, and Known Occurrences in Alaska
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

The Stability of Lead Isotopes from Thorium
MAY 24, I9I7] NATURE ---------------------------------------------------------245 but I Prof. Stefan lYle:yer may be I .have pointed out the unsuitability of makmg some exammatwn of the radiations of the thonum mmerals for age determination or correlation material, and the results he obtains will therefore be and this is particularly so i.n the case of minerals of very great value in deciding this point. the Palreozoic igneous rocks of Langesundfjord, Nor FREDERICK SoDDY. way. Mr. Lawson and myself based our former con Aberdeen, May 14. that lead could not be the end product of thonum largely on analyses of these minerals. How . PRO.F. SoDDY. having given me the privilege of read e:ver, I have recalculated the ratiQs on the assump mg h1s letter m advance, I should like to take the twn that thonum has one-seventh the lead-producing opportunity of directing attention to the geological age power of uranium, and it is satisfactory to find that :>f the thorium minerals of Ceylon, and to a few further when thorium is less than five times a·s abundant statistics bearing on the suggestion that only 35 per uranium, the ratios agree as closely on th'ts calculation cent. of thorium produces a stable isotope of lead. as do the simple lead-ratios. When thorium is more than five times as abundant as uranium neither set I a!ll to my friend, Mr. E. J. Wayland, late of ratios gives any approach to agreement although of Ceylon, for the follow mg prov1s1onal classdicat10n (in order of age) of the the minerals from anv one locality agree am'ong them older rocks of the island :- selves. -

The Rare Earths II
Redis co very of the Elements The Ra re Earth s–The Con fusing Years I A gallery of rare earth scientists and a timeline of their research I I James L. Marshall, Beta Eta 1971 , and Virginia R. Marshall, Beta Eta 2003 , Department of Chemistry, University of North Texas, Denton, TX 76203-5070, [email protected] The rare earths after Mosander. In the pre - vi ou s HEXAGON “Rediscovery” article, 1p we were introduced to the 17 rare earths, found in the f-block and the Group III chemical family of Figure 1. Important scientists dealing with rare earths through the nineteenth century. Johan Gadolin the Periodic Table. Because of a common (1760 –1852) 1g —discovered yttrium (1794). Jöns Jacob Berzelius (1779 –1848) and Martin Heinrich valence electron configuration, the rare earths Klaproth (1743 –1817) 1d —discovered cerium (1803). Carl Gustaf Mosander (1787 –1858) 1p —discovered have similar chemical properties, and their lanthanum (1839), didymium (1840), terbium, and erbium (1843). Jean-Charles deGalissard Marignac chemical separation from one another can be (1817 –1894) 1o —discovered ytterbium (1878) and gadolinium (1880). Per Teodor Cleve (1840 –1905) 1n — difficult. From preparations of the first two rare discovered holmium and thulium (1879). Lars Fredrik Nilson (1840 –1899) 1n —discovered scandium earth element s—yttrium and ceriu m—the (1879). Paul-Émile Lecoq de Boisbaudran (1838 –1912) —discovered samarium (1879) and dysprosium Swedish chemist Carl Gustaf Mosander (Figure (1886). 1b Carl Auer von Welsbach (1858 –1929) 1c —discovered praseodymium and neodymium (1885); 1, 2) was able to separate four additional ele - co-discovered lutetium (1907). -

Abstract Spectroscopic Characterization of Fluorite
ABSTRACT SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR By Carrie Wright This thesis consists of two separate papers on color in fluorite. In the first paper, synthetic fluorites doped with various REEs (10-300 ppm) were analyzed using direct current plasma spectrometry, optical absorption spectroscopy, fluorescence spectrophotometry, and electron paramagnetic resonance spectroscopy before and after receiving 10-25 Mrad of 60Co gamma irradiation. The combined results of these techniques indicate that the irradiation-induced color of the Y-, Gd-, La- and Ce-doped samples are the result of a REE-associated fluorine vacancy that traps two electrons. Divalent samarium may be the cause of the irradiation-induced green color of the Sm- doped sample. In the second paper, fluorite crystals from Bingham, NM, Long Lake, NY, and Westmoreland, NH were similarly investigated to determine the relationship between sectorally zoned trace elements, defects, and color. The results indicate causes of color similar to those in the synthetic samples with the addition of simple F-centers. SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR A Thesis Submitted to the Faculty of Miami University In partial fulfillment of The requirements for the degree of Master of Science Department of Geology By Carrie Wright Miami University Oxford, OH 2002 Advisor_____________________ Dr. John Rakovan Reader______________________ Dr. Hailiang Dong TABLE OF CONTENTS Chapter 1: Introduction to the cause of color in fluorite 1 Manuscript 1-Chapter 2 29 “Spectroscopic investigation of lanthanide doped CaF2 crystals: implications for the cause of color” Manuscript 2-Chapter 3 95 “Spectroscopic characterization of fluorite from Bingham, NM, Long Lake, NY and Westmoreland, NH: relationships between trace element zoning, defects and color ii TABLE OF FIGURES Chapter 1 Figures 21 Figure 1a. -

Stillwellite-(Ce) (Ce, La, Ca)Bsio
Stillwellite-(Ce) (Ce; La; Ca)BSiO5 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Hexagonal. Point Group: 3: As °at rhombohedral crystals, to 4 cm, and massive. Twinning: Observed about [100]. Physical Properties: Cleavage: One imperfect. Fracture: Conchoidal. Hardness = 6.5 D(meas.) = 4.57{4.60 D(calc.) = 4.67 » Optical Properties: Transparent to translucent. Color: Red-brown to pale pink; colorless in thin section. Streak: White. Optical Class: Uniaxial (+) to biaxial (+). ! = 1.765{1.784 ² = 1.780{1.787 2V(meas.) = 0±{6± Cell Data: Space Group: P 31: a = 6.841{6.844 c = 6.700{6.702 Z = 3 X-ray Powder Pattern: Mary Kathleen mine, Australia. 3.43 (s), 2.96 (s), 2.13 (ms), 4.44 (m), 1.864 (m), 2.71 (mw), 2.24 (mw) Chemistry: (1) (2) (1) (2) (1) (2) SiO2 22.40 22.06 La2O3 27.95 19.12 MgO 0.06 UO2 0.22 Ce2O3 33.15 30.82 CaO 0.95 0.34 ThO2 5.41 Pr2O3 1.82 F 0.30 + B2O3 12.23 [13.46] Nd2O3 5.36 H2O 0.85 Al2O3 0.42 Sm2O3 0.34 H2O¡ 0.10 Y2O3 0.74 0.28 Fe2O3 0.18 P2O5 0.67 Total [100.00] [99.26] (1) Mary Kathleen mine, Australia; recalculated to 100.00% after removal of very small amounts of uraninite and apatite determined by separate analysis. (2) Vico volcano, near Vetralla, Italy; by electron microprobe, B2O3 calculated from stoichiometry, original total given as 99.23%; corresponds to (Ce0:50La0:31Nd0:08Th0:05Pr0:03Ca0:02Sm0:01)§=1:00B1:02Si0:97O5: Occurrence: Locally abundant as a metasomatic replacement of metamorphosed calcareous sediments (Mary Kathleen mine, Australia); in alkalic pegmatites in syenite in an alkalic massif (Dara-i-Pioz massif, Tajikistan). -
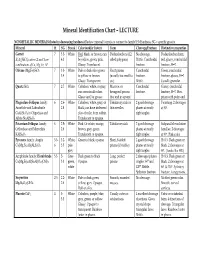
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

RBRC-32 BNL-6835.4 PARITY ODD BUBBLES in HOT QCD D. KHARZEEV in This ~A~Er We Give a Pedawwicalintroduction~0 Recent Work Of
RBRC-32 BNL-6835.4 PARITY ODD BUBBLES IN HOT QCD D. KHARZEEV RIKEN BNL Research Center, Br$ookhauenNational Laboratory, . Upton, New York 11973-5000, USA R.D. PISARSKI Department of Physics, Brookhaven National Laboratoy, Upton, New York 11973-5000, USA M.H.G. TYTGAT Seruice de Physique Th&orique, (7P 225, Uniuersitc4Libre de Bruzelles, B[ud. du !t%iomphe, 1050 Bruxelles, Belgium We consider the topological susceptibility for an SU(N) gauge theory in the limit of a large number of colors, N + m. At nonzero temperature, the behavior of the topological susceptibility depends upon the order of the reconfining phrrse transition. The meet interesting possibility is if the reconfining transition, at T = Td, is of second order. Then we argue that Witten’s relation implies that the topological suscepti~lfity vanishes in a calculable fdion at Td. Ae noted by Witten, this implies that for sufficiently light quark messes, metaetable etates which act like regions of nonzero O — parity odd bubbles — can arise at temperatures just below Td. Experimentally, parity odd bubbles have dramatic signature% the rI’ meson, and especially the q meson, become light, and are copiously produced. Further, in parity odd bubbles, processes which are normally forbidden, such as q + rr”ro, are allowed. The most direct way to detect parity violation is by measuring a parity odd global seymmetry for charged pions, which we define. 1 Introduction In this .-~a~er we give a Pedawwicalintroduction~0 recent work of ours? We I consider an SU(IV) gau”ge t~e~ry in the limit of a large number of colors, N + co, This is, of course, a familiar limit? We use the large N expansion I to investigate the behavior of the theory at nonzero temperature, especially for the topological susceptibility. -

Winter 1998 Gems & Gemology
WINTER 1998 VOLUME 34 NO. 4 TABLE OF CONTENTS 243 LETTERS FEATURE ARTICLES 246 Characterizing Natural-Color Type IIb Blue Diamonds John M. King, Thomas M. Moses, James E. Shigley, Christopher M. Welbourn, Simon C. Lawson, and Martin Cooper pg. 247 270 Fingerprinting of Two Diamonds Cut from the Same Rough Ichiro Sunagawa, Toshikazu Yasuda, and Hideaki Fukushima NOTES AND NEW TECHNIQUES 281 Barite Inclusions in Fluorite John I. Koivula and Shane Elen pg. 271 REGULAR FEATURES 284 Gem Trade Lab Notes 290 Gem News 303 Book Reviews 306 Gemological Abstracts 314 1998 Index pg. 281 pg. 298 ABOUT THE COVER: Blue diamonds are among the rarest and most highly valued of gemstones. The lead article in this issue examines the history, sources, and gemological characteristics of these diamonds, as well as their distinctive color appearance. Rela- tionships between their color, clarity, and other properties were derived from hundreds of samples—including such famous blue diamonds as the Hope and the Blue Heart (or Unzue Blue)—that were studied at the GIA Gem Trade Laboratory over the past several years. The diamonds shown here range from 0.69 to 2.03 ct. Photo © Harold & Erica Van Pelt––Photographers, Los Angeles, California. Color separations for Gems & Gemology are by Pacific Color, Carlsbad, California. Printing is by Fry Communications, Inc., Mechanicsburg, Pennsylvania. © 1998 Gemological Institute of America All rights reserved. ISSN 0016-626X GIA “Cut” Report Flawed? The long-awaited GIA report on the ray-tracing analysis of round brilliant diamonds appeared in the Fall 1998 Gems & Gemology (“Modeling the Appearance of the Round Brilliant Cut Diamond: An Analysis of Brilliance,” by T. -

Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H
Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Cover Photo: Various alkaline ultramafic rocks showing porphyritic, brecciated, and aphanitic textures, in contact with host limestone and altered dike texture. From left to right: • Davidson North dike, Davidson core, YH-04, 800 ft depth. Lamprophyre with calcite veins, containing abundant rutile. • Coefield area, Billiton Minner core BMN 3. Intrusive breccia with lamprophyric (al- nöite) matrix. • Maple Lake area, core ML-1, 416 ft depth. Lamprophyre intrusive. • Maple Lake area, core ML-2, 513 ft depth. Lamprophyre (bottom) in contact with host limestone (top). Kentucky Geological Survey University of Kentucky, Lexington Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Our Mission The Kentucky Geological Survey is a state-supported research center and public resource within the University of Kentucky. Our mission is to sup- port sustainable prosperity of the commonwealth, the vitality of its flagship university, and the welfare of its people. We do this by conducting research and providing unbiased information about geologic resources, environmen- tal issues, and natural hazards affecting Kentucky. Earth Resources—Our Common Wealth www.uky.edu/kgs © 2019 University of Kentucky For further information contact: Technology Transfer Officer Kentucky Geological Survey 228 Mining and Mineral Resources Building University of Kentucky Lexington, KY 40506-0107 Technical Level General Intermediate Technical Statement of Benefit to Kentucky Rare earth elements are used in many applications in modern society, from cellphones to smart weapons systems. -

Andradite in Andradite Unusual Growth Zoning in Beryl
Editor Nathan Renfro Contributing Editors Elise A. Skalwold and John I. Koivula Andradite in Andradite ity, but size was not what made it special. As shown in fig- Recently we had the opportunity to examine a dramatic ure 1, close examination of one of the polished crystal faces iridescent andradite fashioned by Falk Burger (Hard Works, revealed a bright reddish orange “hot spot” in the center, Tucson, Arizona) from a crystal originating from the caused by an iridescent inclusion of andradite with a dif- Tenkawa area of Nara Prefecture in Japan. Known as “rain- ferent crystallographic orientation than its host. As seen bow” andradite, this material was previously reported in in figure 2, the inclusion’s different orientation caused the iridescence of the rhomb-shaped “hot spot” to appear and Gems & Gemology (T. Hainschwang and F. Notari, “The cause of iridescence in rainbow andradite from Nara, disappear as the light source was passed over the crystal’s Japan,” Winter 2006, pp. 248–258). The specimen was surface. To see the iridescence from both the host and in- unique for its genesis and optical phenomenon. clusion at the same time, two light sources from opposite Weighing 16.79 ct and measuring 15.41 × 13.86 × 10.49 directions must be used due to the different crystallo- mm, the andradite was very large for its species and local- graphic orientation of the host and inclusion. This elusive optical phenomenon made this Japanese andradite crystal extremely interesting for any aspiring inclusionist. John I. Koivula Figure 1. This 16.79 ct Japanese andradite garnet GIA, Carlsbad exhibits a very unusual rhomb-shaped “hot spot” below the surface of one crystal face. -

Crystallographic Study of Uranium-Thorium Bearing Minerals in Tranomaro, South-East Madagascar
Journal of Minerals and Materials Characterization and Engineering, 2013, 1, 347-352 Published Online November 2013 (http://www.scirp.org/journal/jmmce) http://dx.doi.org/10.4236/jmmce.2013.16053 Crystallographic Study of Uranium-Thorium Bearing Minerals in Tranomaro, South-East Madagascar Frank Elliot Sahoa1, Naivo Rabesiranana1*, Raoelina Andriambololona1, Nicolas Finck2, Christian Marquardt2, Hörst Geckeis2 1Institut National des Sciences et Techniques Nucléaires (Madagascar-INSTN), Antananarivo, Madagascar 2Institute of Nuclear Waste and Disposal (INE), Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany Email: *[email protected] Received September 20, 2013; revised October 21, 2013; accepted November 5, 2013 Copyright © 2013 Frank Elliot Sahoa et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Studies are undertaken to characterize the uranium and thorium minerals of south-east Madagascar. Seven selected uranothorianite bearing pyroxenites samples from old abandoned uranium quarries in Tranomaro, south Amboasary, Madagascar (46˚28'00"E, 24˚36'00"S) have been collected. To determine the mineral micro-structure, they were inves- tigated for qualitative identification of crystalline compounds by using X-ray powder diffraction analytical method (XRD). Results showed that the uranium and thorium compounds, as minor elements, were present in various crystal- line structures. Thorium, as thorianite, is present in a simple ThO2 cubic crystalline system, whereas the uranium com- ponent of the Tranomaro uranothorianite samples is oxide-based and is a mixture of complex oxidation states and crys- talline systems. Generally called uraninite, its oxide compounds are present in more than eight phases. -

The Eyes of Africa
The Eyes of Africa Where is it from? Erongo Mountain, Erongo Region, Namibia What are its dimensions? H: 22.8 in W: 13.3 in D: 10.2 in How much does it weight? 64.3 Pounds (29.2 kilos) What is this mineral made of? Fluorite & Quartz What is Fluorite (CaF2)? Named in 1797 by Carlo Antonio Galeani Napione from the Latin, fluere = “to flow” (for its use as a flux). The term fluores- cence is derived from fluorite, which will often markedly exhib- it this effect. The element fluorine also derives its name from fluorite. What is Quartz (SiO2)? Quartz has been known and appreciated since prehistoric times. Kristallos is the most ancient name known for quartz, recorded by Theophrastus in about 300-325 BCE. The root words κρύοσ (ice cold) and στέλλειυ (to contract) suggest the ancient belief that kristallos was permanently solidified ice. Brief Description: This specimen is known as “The Eyes of Africa.” It is composed of several white, semi-translucent quartz crystals and large, green and black Alien Eye Fluorites. It was recovered from the Erongo Region of Namibia in 2007. Alien Eyes are a unique and unusual subset of fluorite that differ- entiates itself with its vivid green color and black outer zones that create a diamond shape at each crystal’s center. They also have a naturally formed, complex crystal habit in the form of cuboctahedra. With light, Alien Eye fluorites glow with an incredible otherworldly quality which was what inspired their name. The total number of Alien Eye Fluorites recovered from the find is low, amounting to less than 30 fine specimens, due to the small pocket size and the fact that there was only one single discovery.