Abstract Spectroscopic Characterization of Fluorite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barium Trifluoromethanesulfonate
CXBA083 - BARIUM TRIFLUOROMETHANESULFONATE BARIUM TRIFLUOROMETHANESULFONATE Safety Data Sheet CXBA083 Date of issue: 07/28/2017 Version: 1.0 SECTION 1: Identification 1.1. Product identifier Product name : BARIUM TRIFLUOROMETHANESULFONATE Product code : CXBA083 Product form : Substance Physical state : Solid Formula : C2BaF6O6S2 Synonyms : BARIUM TRIFLATE METHANESULFONIC ACID, 1,1,1-TRIFLUORO-, BARIUM SALT Chemical family : METAL COMPOUND 1.2. Recommended use of the chemical and restrictions on use Recommended use : Chemical intermediate For research and industrial use only 1.3. Details of the supplier of the safety data sheet GELEST, INC. 11 East Steel Road Morrisville, PA 19067 USA T 215-547-1015 - F 215-547-2484 - (M-F): 8:00 AM - 5:30 PM EST [email protected] - www.gelest.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 (USA); +1 703-527-3887 (International) SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Skin corrosion/irritation Category 2 H315 Serious eye damage/eye irritation Category 2 H319 Full text of H statements : see section 16 2.2. Label elements GHS-US labeling Hazard pictograms (GHS-US) : GHS07 Signal word (GHS-US) : Warning Hazard statements (GHS-US) : H315 - Causes skin irritation H319 - Causes serious eye irritation Precautionary statements (GHS-US) : P280 - Wear protective gloves/protective clothing/eye protection/face protection P264 - Wash hands thoroughly after handling P302+P352 - If on skin: Wash with plenty of soap and water P332+P313 - If skin irritation occurs: Get medical advice/attention P305+P351+P338 - IF IN EYES: Rinse cautiously with water for several minutes. -

A New Gamma Camera for Positron Emission Tomography
INIS-mf—11552 A new gamma camera for Positron Emission Tomography NL89C0813 P. SCHOTANUS A new gamma camera for Positron Emission Tomography A new gamma camera for Positron Emission Tomography PROEFSCHRIFT TER VERKRIJGING VAN DE GRAAD VAN DOCTOR AAN DE TECHNISCHE UNIVERSITEIT DELFT, OP GEZAG VAN DE RECTOR MAGNIFICUS, PROF.DRS. P.A. SCHENCK, IN HET OPENBAAR TE VERDEDIGEN TEN OVERSTAAN VAN EEN COMMISSIE, AANGEWEZEN DOOR HET COLLEGE VAN DECANEN, OP DINSDAG 20 SEPTEMBER 1988TE 16.00 UUR. DOOR PAUL SCHOTANUS '$ DOCTORANDUS IN DE NATUURKUNDE GEBOREN TE EINDHOVEN Dit proefschrift is goedgekeurd door de promotor Prof.dr. A.H. Wapstra s ••I Sommige boeken schijnen geschreven te zijn.niet opdat men er iets uit zou leren, maar opdat men weten zal, dat de schrijver iets geweten heeft. Goethe Contents page 1 Introduction 1 2 Nuclear diagnostics as a tool in medical science; principles and applications 2.1 The position of nuclear diagnostics in medical science 2 2.2 The detection of radiation in nuclear diagnostics: 5 standard techniques 2.3 Positron emission tomography 7 2.4 Positron emitting isotopes 9 2.5 Examples of radiodiagnostic studies with PET 11 2.6 Comparison of PET with other diagnostic techniques 12 3 Detectors for positron emission tomography 3.1 The absorption d 5H keV annihilation radiation in solids 15 3.2 Scintillators for the detection of annihilation radiation 21 3.3 The detection of scintillation light 23 3.4 Alternative ways to detect annihilation radiation 28 3-5 Determination of the point of annihilation: detector geometry, -

Cryogenic Selective Surfaces NIAC Phase II Study Final Report
Cryogenic Selective Surfaces NIAC Phase II Study Final Report September 2018 Robert C. Youngquist, Ph.D. Mark A. Nurge, Ph.D. Wesley L. Johnson Tracy L. Gibson, Ph.D. Cryogenic Selective Surfaces NIAC Phase II Study September 2018 Final Report i. Executive Summary There are many challenges involved in deep-space exploration, but several of these can be mitigated, or even solved, by the development of a coating that reflects most of the Sun’s energy, yet still provides far-infrared heat emission. Such a coating would allow non-heat-generating objects in space to reach cryogenic temperatures without using an active cooling system. This would benefit deep-space sensors that require low temperatures, such as the James Webb Telescope focal plane array. It would also allow the use of superconductors in deep space, which could lead to magnetic energy storage rings, lossless power delivery, or perhaps a large-volume magnetic shield against galactic cosmic radiation. However, perhaps the most significant enablement achieved from such a coating would be the long- term, deep space storage of cryogenic liquids, such as liquid oxygen (LOX). In our Phase I NIAC study, we realized that a combination of scattering particles and a silver backing could yield a highly effective, very broadband, reflector that could potentially reflect more than 99.9% of the Sun’s irradiant power. We developed a sophisticated model of this reflector and theoretically showed that cryogenic temperatures could be achieved in deep space at one astronomical unit (1 AU) from the Sun. We showed how this new reflector could minimize heat conduction into the cryogenic tanks by coating the tank support struts. -

Molecular Dynamics Simulation Superionic Conductor Baf2
MOLECULAR DYNAMICS SIMULATION OF SUPERIONIC CONDUCTOR BaF2 A. K. IVANOV-SHITZ, AND S. KH. AITYAN Institute of Crystallography, USSR Acad. Sci., Moscow INTRODUCTION The superionic conductors are of interest from the standpoint of both their intrinsic scientific problems of solid state physics and their technolog- ical importance. It should be noted that these systems are very difficult to treat using simple analytic theory because they have a high degree of di- sorder. On the other hand, the computer simulation is suited for studying such strong anharmonicity systems and we report on how molecular dy- namics simulation has been used to investigate superionic conductors. It should be stressed that fluoride conductors with the fluorite struc- ture appear to be a suitable model for studying features of ionic transport in solids, because they have a simple crystal structure.' Fluoride materials such as Ca, Sr, Ba, Pb fluorides (as well as Sr chloride -SrCl2) crystallize in the fluorite structure. These compounds are ordinary ionic conductors at low temperatures and only just before melting point they undergo the so-called diffuse transition, accompanied by a significant increase in con- ductivity and a pronounced peak in specific heat.2 The new high-tempera- ture modification is retained up to the melting poin Tn,. At present structural and transport characteristics of BaF2 at high temperatures appear to be studied insufficiently. That is why it was rather important to study specific features of the crystal structure and ion trans- port just in this compound, in order to confirm the existence of the super- ionic state. To do this we carried out a computer simulation by the molecular dy- namics technique. -
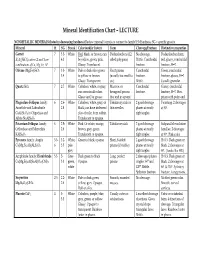
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product identifier Barium Fluoride (BaF2) targets Other means of identification SDS number 2GV Materion Code 2GV CAS number 7787-32-8 Synonyms BARIUM FLUORIDE * Barium difluoride (as F) * Barium difluoride Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name Materion Advanced Chemicals Inc. Address 407 N 13th Street 1316 W. St. Paul Avenue Milwaukee, WI 53233 United States Telephone 414.212.0257 E-mail [email protected] Contact person Noreen Atkinson Emergency phone number Chemtrec 800.424.9300 2. Hazard(s) identification Physical hazards Not classified. Health hazards Acute toxicity, oral Category 3 Acute toxicity, inhalation Category 4 Serious eye damage/eye irritation Category 2 Reproductive toxicity Category 2 Specific target organ toxicity, single exposure Category 3 respiratory tract irritation Specific target organ toxicity, repeated Category 1 exposure Environmental hazards Not classified. OSHA defined hazards Not classified. Label elements Hazard symbol None. Signal word None. Hazard statement The Material as sold in solid form is generally not considered hazardous. However, if the process involves grinding, melting, cutting or any other process that causes a release of dust or fumes, hazardous levels of airborne particulate could be generated. Precautionary statement Prevention Wear protective gloves/protective clothing/eye protection/face protection. Wash thoroughly after handling. Response Call a physician if symptoms develop or persist. Storage Store in a well-ventilated place. Keep container tightly closed. Disposal Dispose of contents/container in accordance with local/regional/national/international regulations. Hazard(s) not otherwise None known. classified (HNOC) Supplemental information None. Material name: Barium Fluoride (BaF2) targets SDS US 2GV Version #: 01 Issue date: 01-19-2018 1 / 6 3. -

Winter 1998 Gems & Gemology
WINTER 1998 VOLUME 34 NO. 4 TABLE OF CONTENTS 243 LETTERS FEATURE ARTICLES 246 Characterizing Natural-Color Type IIb Blue Diamonds John M. King, Thomas M. Moses, James E. Shigley, Christopher M. Welbourn, Simon C. Lawson, and Martin Cooper pg. 247 270 Fingerprinting of Two Diamonds Cut from the Same Rough Ichiro Sunagawa, Toshikazu Yasuda, and Hideaki Fukushima NOTES AND NEW TECHNIQUES 281 Barite Inclusions in Fluorite John I. Koivula and Shane Elen pg. 271 REGULAR FEATURES 284 Gem Trade Lab Notes 290 Gem News 303 Book Reviews 306 Gemological Abstracts 314 1998 Index pg. 281 pg. 298 ABOUT THE COVER: Blue diamonds are among the rarest and most highly valued of gemstones. The lead article in this issue examines the history, sources, and gemological characteristics of these diamonds, as well as their distinctive color appearance. Rela- tionships between their color, clarity, and other properties were derived from hundreds of samples—including such famous blue diamonds as the Hope and the Blue Heart (or Unzue Blue)—that were studied at the GIA Gem Trade Laboratory over the past several years. The diamonds shown here range from 0.69 to 2.03 ct. Photo © Harold & Erica Van Pelt––Photographers, Los Angeles, California. Color separations for Gems & Gemology are by Pacific Color, Carlsbad, California. Printing is by Fry Communications, Inc., Mechanicsburg, Pennsylvania. © 1998 Gemological Institute of America All rights reserved. ISSN 0016-626X GIA “Cut” Report Flawed? The long-awaited GIA report on the ray-tracing analysis of round brilliant diamonds appeared in the Fall 1998 Gems & Gemology (“Modeling the Appearance of the Round Brilliant Cut Diamond: An Analysis of Brilliance,” by T. -

Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H
Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Cover Photo: Various alkaline ultramafic rocks showing porphyritic, brecciated, and aphanitic textures, in contact with host limestone and altered dike texture. From left to right: • Davidson North dike, Davidson core, YH-04, 800 ft depth. Lamprophyre with calcite veins, containing abundant rutile. • Coefield area, Billiton Minner core BMN 3. Intrusive breccia with lamprophyric (al- nöite) matrix. • Maple Lake area, core ML-1, 416 ft depth. Lamprophyre intrusive. • Maple Lake area, core ML-2, 513 ft depth. Lamprophyre (bottom) in contact with host limestone (top). Kentucky Geological Survey University of Kentucky, Lexington Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Our Mission The Kentucky Geological Survey is a state-supported research center and public resource within the University of Kentucky. Our mission is to sup- port sustainable prosperity of the commonwealth, the vitality of its flagship university, and the welfare of its people. We do this by conducting research and providing unbiased information about geologic resources, environmen- tal issues, and natural hazards affecting Kentucky. Earth Resources—Our Common Wealth www.uky.edu/kgs © 2019 University of Kentucky For further information contact: Technology Transfer Officer Kentucky Geological Survey 228 Mining and Mineral Resources Building University of Kentucky Lexington, KY 40506-0107 Technical Level General Intermediate Technical Statement of Benefit to Kentucky Rare earth elements are used in many applications in modern society, from cellphones to smart weapons systems. -

Andradite in Andradite Unusual Growth Zoning in Beryl
Editor Nathan Renfro Contributing Editors Elise A. Skalwold and John I. Koivula Andradite in Andradite ity, but size was not what made it special. As shown in fig- Recently we had the opportunity to examine a dramatic ure 1, close examination of one of the polished crystal faces iridescent andradite fashioned by Falk Burger (Hard Works, revealed a bright reddish orange “hot spot” in the center, Tucson, Arizona) from a crystal originating from the caused by an iridescent inclusion of andradite with a dif- Tenkawa area of Nara Prefecture in Japan. Known as “rain- ferent crystallographic orientation than its host. As seen bow” andradite, this material was previously reported in in figure 2, the inclusion’s different orientation caused the iridescence of the rhomb-shaped “hot spot” to appear and Gems & Gemology (T. Hainschwang and F. Notari, “The cause of iridescence in rainbow andradite from Nara, disappear as the light source was passed over the crystal’s Japan,” Winter 2006, pp. 248–258). The specimen was surface. To see the iridescence from both the host and in- unique for its genesis and optical phenomenon. clusion at the same time, two light sources from opposite Weighing 16.79 ct and measuring 15.41 × 13.86 × 10.49 directions must be used due to the different crystallo- mm, the andradite was very large for its species and local- graphic orientation of the host and inclusion. This elusive optical phenomenon made this Japanese andradite crystal extremely interesting for any aspiring inclusionist. John I. Koivula Figure 1. This 16.79 ct Japanese andradite garnet GIA, Carlsbad exhibits a very unusual rhomb-shaped “hot spot” below the surface of one crystal face. -

The Eyes of Africa
The Eyes of Africa Where is it from? Erongo Mountain, Erongo Region, Namibia What are its dimensions? H: 22.8 in W: 13.3 in D: 10.2 in How much does it weight? 64.3 Pounds (29.2 kilos) What is this mineral made of? Fluorite & Quartz What is Fluorite (CaF2)? Named in 1797 by Carlo Antonio Galeani Napione from the Latin, fluere = “to flow” (for its use as a flux). The term fluores- cence is derived from fluorite, which will often markedly exhib- it this effect. The element fluorine also derives its name from fluorite. What is Quartz (SiO2)? Quartz has been known and appreciated since prehistoric times. Kristallos is the most ancient name known for quartz, recorded by Theophrastus in about 300-325 BCE. The root words κρύοσ (ice cold) and στέλλειυ (to contract) suggest the ancient belief that kristallos was permanently solidified ice. Brief Description: This specimen is known as “The Eyes of Africa.” It is composed of several white, semi-translucent quartz crystals and large, green and black Alien Eye Fluorites. It was recovered from the Erongo Region of Namibia in 2007. Alien Eyes are a unique and unusual subset of fluorite that differ- entiates itself with its vivid green color and black outer zones that create a diamond shape at each crystal’s center. They also have a naturally formed, complex crystal habit in the form of cuboctahedra. With light, Alien Eye fluorites glow with an incredible otherworldly quality which was what inspired their name. The total number of Alien Eye Fluorites recovered from the find is low, amounting to less than 30 fine specimens, due to the small pocket size and the fact that there was only one single discovery. -

PET--The History Behind the Technology
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Senior Thesis Projects, 1993-2002 College Scholars 2002 PET--The History Behind the Technology Michael A. Steiner Follow this and additional works at: https://trace.tennessee.edu/utk_interstp2 Recommended Citation Steiner, Michael A., "PET--The History Behind the Technology" (2002). Senior Thesis Projects, 1993-2002. https://trace.tennessee.edu/utk_interstp2/107 This Project is brought to you for free and open access by the College Scholars at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Senior Thesis Projects, 1993-2002 by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. PET - The history Behind the Technology College Scholars Senior Project Michael A. Steiner May 6,2002 Committee Members: Dr. George Kabalka Dr. Gary T. Smith Raymond Trotta Brief Overview Positron Emission Tomography, commonly known by the acronym PET, is a branch of nuclear medicine that has come to be an important imaging diagnostic in the medical field. PET relies on the use ofradioactive isotopes to visualize physiological processes ofthe body. An isotope of an element has the same number of protons as its counterpart but di ffers in the nun1ber ofneutrons. The difference in neutrons gives the isotope a different mass number and can also provide instability to the element or molecule derived from the isotope. There are two fundamental differences between traditional nuclear medicine and PET. First, the radiotracers that are utilized in PET emit positrons. Also, there is difference in the process by which data is acquired and reconstructed into an image. -

BARIUM FLUORIDE * * *Section 2
Material Safety Data Sheet Material Name: BARIUM FLUORIDE MSDS ID: BTI02400 * * *Section 1 - PRODUCT AND COMPANY IDENTIFICATION* * * Material Name: BARIUM FLUORIDE Manufacturer Information BASSTECH INTERNATIONAL LLC 400 Kelby St. Fax: (201) 569-7511 Fort Lee, NJ 07024 Emergency #: CHEMTREC: 800-424-9300 Phone: (201) 569-8686 Email: [email protected] Chemical Family inorganic, salt Synonyms BARIUM DIFLUORIDE; BARIUM FLUORIDE (BAF2); BAF2; RTECS: CQ9100000 * * *Section 2 - HAZARDS IDENTIFICATION* * * EMERGENCY OVERVIEW Color: colorless to white Physical Form: crystalline powder Odor: odorless Health Hazards: harmful if swallowed POTENTIAL HEALTH EFFECTS Inhalation Short Term: same as effects reported in other routes of exposure, irritation (possibly severe), nausea, difficulty breathing, asthma, lung congestion Long Term: same as effects reported in long term ingestion, lung damage Skin Short Term: same as effects reported in short term ingestion, irritation (possibly severe) Long Term: same as effects reported in short term exposure Eye Short Term: irritation (possibly severe) Long Term: same as effects reported in short term exposure Ingestion Short Term: burns, rash, metallic taste, ringing in the ears, digestive disorders, difficulty breathing, irregular heartbeat, headache, drowsiness, dizziness, tingling sensation, visual disturbances, dilated pupils, bluish skin color, kidney damage, paralysis, convulsions, coma Long Term: no information on significant adverse effects * * *Section 3 - COMPOSITION / INFORMATION ON INGREDIENTS* * * ____________________________________________________________ Page 1 of 8 Issue Date: 03/18/2014 Revision: 1.1300 Print Date: 03/18/2014 Material Safety Data Sheet Material Name: BARIUM FLUORIDE MSDS ID: BTI02400 CAS # Component Percent Symbol(s) Risk Phrase(s) 7787-32-8 BARIUM FLUORIDE 100.0 Xn R:20/22 232-108-0 Component Related Regulatory Information This product may be regulated, have exposure limits or other information identified as the following: Barium compounds, inorganic, Fluorides, Barium compounds, n.o.s.