Foraging Activity and Control of Termites In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Tulu Kapi Nyota Min Ltd '09 Technical
- Key Features - Exploration - Introduction - Mineralisation - Project Description, General Infrastructure and - Distribution of Mineralistation Accessibility - Deposit Type - Topography, Climate and Vegetation - Sampling Method and Approach - Legal Aspects and Tenure - Sample Preparation, Analysis and Security - Environmental Requirements - Mineralogical Studies and Mineral Processing - Country History - Data Verification and QA/QC - Country Profile and Economy - Adjacent Properties and Competitor Companies - Mining Sector of the Economy - Modelling and Mineral Resource Estimation IN THIS DOCUMENT - Historical Exploration and Operations in the Tulu - Conclusions Investors Report on the Kapi Area - References Tulu Kapi Gold Project, Ethiopia - Regional Geological Setting th - Local Geology as at 30 September 2009 KEY FEATURES Compliance: Venmyn utilises a comprehensive checklist incorporating all internationally required compliance requirements, in particular the Canadian National Instrument 43-101 and SAMREC/SAMVAL Codes for public reporting of mineral assets. The information quoted in this Prospectivity Review has been scrutinised in terms of this checklist and prepared for investors according to the principles of open and transparent disclosure embodied in the underlying codes for mineral resources reporting. Qualified Persons: Mr.Andy Clay, M.Sc. (Geol), M.Sc. (Min. Eng.), Dip.Bus.M., Pr.Sci.Nat., MSAIMM, FAusIMM, FGSSA,AAPG, M.Inst.D. Mr. Neil Mc Kenna, M.Sc. (Geol), Pr.Sci.Nat., MSAIMM, MGSSA, MIASSA, M.Inst.D. Mr. Richard Tayelor, B.Sc. Hons (Geol). MGSSA. Effective Date: 30th September 2009. Prepared For: Nyota Minerals Limeted (Nyota), previously Dwyka Resources Limited (Dwyka). Purpose: Review of the prospectivity and technical merits of the Tulu Kapi Gold Project in Ethiopia. Sources of Information: Public domain information as listed in the reference list, Nyota, Dwyka and Minerva Resources PLC internal reports and, presentations and Hellman & Schofield (Pty) Ltd. -

Treatise on the Isoptera of the World Kumar
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by American Museum of Natural History Scientific Publications KRISHNA ET AL.: ISOPTERA OF THE WORLD: 7. REFERENCES AND INDEX7. TREATISE ON THE ISOPTERA OF THE WORLD 7. REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA, AND MICHAEL S. ENGEL A MNH BULLETIN (7) 377 2 013 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY TREATISE ON THE ISOPTERA OF THE WORLD VolUME 7 REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192 AND MICHAEL S. ENGEL Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192; Division of Entomology (Paleoentomology), Natural History Museum and Department of Ecology and Evolutionary Biology 1501 Crestline Drive, Suite 140 University of Kansas, Lawrence, Kansas 66045 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 377, 2704 pp., 70 figures, 14 tables Issued April 25, 2013 Copyright © American Museum of Natural History 2013 ISSN 0003-0090 2013 Krishna ET AL.: ISOPtera 2435 CS ONTENT VOLUME 1 Abstract...................................................................... 5 Introduction.................................................................. 7 Acknowledgments . 9 A Brief History of Termite Systematics ........................................... 11 Morphology . 44 Key to the -

Diversity and Abundance of Subterranean Termites in South India
Srinivasa Murthy, K. Available Ind. J. Pure online App. Biosci.at www.ijpab.com (2020) 8(5), 141 -149 ISSN: 2582 – 2845 DOI: http://dx.doi.org/10.18782/2582-2845.8193 ISSN: 2582 – 2845 Ind. J. Pure App. Biosci. (2020) 8(5), 141-149 Research Article Peer-Reviewed, Refereed, Open Access Journal Diversity and abundance of Subterranean Termites in South India K. Srinivasa Murthy* National Bureau of Agricultural Insect Resources, P B No. 2491, H A Farm Post, Bellary Road Bangalore - 560 024, Karnataka, India *Corresponding Author E-mail: [email protected] Received: 7.07.2020 | Revised: 12.08.2020 | Accepted: 20.08.2020 ABSTRACT The abundance and diversity of subterranean termites was studied in the states of Andhra Pradesh, Keralae, Karnataka and Tamilnadu. Fifteen species of termites belonging to subfamilies Apicotermitinae, Kalotermitidae, Macrotermitinae and Nasutitermitinae, were recorded. The fungus growing termites (Macrotermitinae) accounted for 66.66% abundance, across the states. The Apicotermitinae (soil feeders) and Kalotermitidae (dry wood termites) registered 6.62% each and the dry wood termites (Nasutitermitinae) recorded 20.1% abundance. Among the different species of termites, Odontermes obesus, was more predominant (15.62%) than others. The cropping pattern, soil type and topography predisposed the abundance and diversity of termites. Keywords: Abundance, Cropping pattern, Diversity, Macrotermitinae. INTRODUCTION Ali, et al., 2013) as they play a vital role in Termites (Isoptera) are considered as the most recycling of plant materials and wood, abundant invertebrates and represent up to modifying and improving the soil condition 95% of soil insect biomass show an elaborated and composition, and providing food for other morphology and complex behaviour (Wang, et animals (Ackerman et al. -

Termiticidal Activity of Bifenthrin and Fipronil Against Mound Building Termite Odontotermes Redemanni Wasmann
Annals of Sri Lanka Department of Agriculture 2017. 19: 104 - 111 TERMITICIDAL ACTIVITY OF BIFENTHRIN AND FIPRONIL AGAINST MOUND BUILDING TERMITE ODONTOTERMES REDEMANNI WASMANN N.K. HAPUKOTUWA1 AND S. PERERA2 1 Plant Protection Service, Gannoruwa, Sri Lanka 2 Plant Protection Service Subunit, Bombuwala, Sri Lanka ABSTRACT Bifenthrin 10% SC (Maxxthor) and Fipronil 25% EC (Premise) obtained from two new sources were tested during 2014 to determine their termiticidal activity against the mound building termite Odontotermes redemanni Wasmann. Termitariaat open landscape in School of Agriculture, Kundasale, Kandy was randomly as the source of termite. Two new chemicals (Maxxthor and Premise), two reference chemicals (Biflex® and Agenda®) and water (control) were used as treatments with three replicates. Tendried sticks (60 cm long and three cm diameter) of Kapok (Ceibapentandra) were treated and inserted into each termitarium allowing the termites to feed on. Sticks were removed at two time intervals: four and eight weeks and weighed separately to measure the wood consumption. Analysis of variance showed highly significant differences (p=0.0001) in wood consumption among the treatments: chemicals and water treated termitaria. Maximum wood consumption (88.4g/8 weeks) was recorded in water treated termitaria. However no significant difference in wood consumption was observed between chemically treated termitaria. Both chemicals irrespective of their active ingredients, formulation or country of origin, performed equally against O. Redemanni revealing that they are appropriate termiticides to control mound building termite problem in Sri Lanka. Key words: Chemical control, Termites, Termitaria, Termiticide, wood consumption. INTRODUCTION Termites are abundant and diverse throughout the world (Donald and Dweight, 1970; Maayiem et al., 2012). -

Aalborg Universitet Restructuring State and Society Ethnic
Aalborg Universitet Restructuring State and Society Ethnic Federalism in Ethiopia Balcha, Berhanu Publication date: 2007 Document Version Publisher's PDF, also known as Version of record Link to publication from Aalborg University Citation for published version (APA): Balcha, B. (2007). Restructuring State and Society: Ethnic Federalism in Ethiopia. SPIRIT. Spirit PhD Series No. 8 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. ? Users may download and print one copy of any publication from the public portal for the purpose of private study or research. ? You may not further distribute the material or use it for any profit-making activity or commercial gain ? You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us at [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from vbn.aau.dk on: November 29, 2020 SPIRIT Doctoral Programme Aalborg University Kroghstraede 3-3.237 DK-9220 Aalborg East Phone: +45 9940 9810 Mail: [email protected] Restructuring State and Society: Ethnic Federalism in Ethiopia Berhanu Gutema Balcha SPIRIT PhD Series Thesis no. 8 ISSN: 1903-7783 © 2007 Berhanu Gutema Balcha Restructuring State and Society: Ethnic Federalism in Ethiopia SPIRIT – Doctoral Programme Aalborg University Denmark SPIRIT PhD Series Thesis no. -

Smithsonian Miscellaneous Collections
Ubr.C-ff. SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 143, NO. 3 SUPPLEMENT TO THE ANNOTATED, SUBJECT-HEADING BIBLIOGRAPHY OF TERMITES 1955 TO I960 By THOMAS E. SNYDER Honorary Research Associate Smithsonian Institution (Publication 4463) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION DECEMBER 29, 1961 SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 143, NO. 3 SUPPLEMENT TO THE ANNOTATED, SUBJECT-HEADING BIBLIOGRAPHY OF TERMITES 1955 TO 1960 By THOMAS E. SNYDER Honorary Research Associate Smithsonian Institution ><%<* Q (Publication 4463) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION DECEMBER 29, 1961 PORT CITY PRESS, INC. BALTIMORE, NID., U. S. A. CONTENTS Pagre Introduction i Acknowledgments i List of subject headings 2 Subject headings 3 List of authors and titles 72 Index 115 m SUPPLEMENT TO THE ANNOTATED, SUBJECT-HEADING BIBLIOGRAPHY OF TERMITES 1955 TO 1960 By THOMAS E. SNYDER Honorary Research Associate Smithsonian Institution INTRODUCTION On September 25, 1956, an "Annotated, Subject-Heading Bibliography of Ter- mites 1350 B.C. to A.D. 1954," by Thomas E. Snyder, was published as volume 130 of the Smithsonian Miscellaneous Collections. A few 1955 papers were included. The present supplement covers publications from 1955 through i960; some 1961, as well as some earlier, overlooked papers, are included. A total of 1,150 references are listed under authors and tides, and 2,597 references are listed under subject headings, the greater number being due to cross references to publications covering more than one subject. New subject headings are Radiation and Toxicology. ACKNOWLEDGMENTS The publication of this bibliography was made possible by a grant from the National Science Foundation, Washington, D.C. -

Ethiopia COI Compilation
BEREICH | EVENTL. ABTEILUNG | WWW.ROTESKREUZ.AT ACCORD - Austrian Centre for Country of Origin & Asylum Research and Documentation Ethiopia: COI Compilation November 2019 This report serves the specific purpose of collating legally relevant information on conditions in countries of origin pertinent to the assessment of claims for asylum. It is not intended to be a general report on human rights conditions. The report is prepared within a specified time frame on the basis of publicly available documents as well as information provided by experts. All sources are cited and fully referenced. This report is not, and does not purport to be, either exhaustive with regard to conditions in the country surveyed, or conclusive as to the merits of any particular claim to refugee status or asylum. Every effort has been made to compile information from reliable sources; users should refer to the full text of documents cited and assess the credibility, relevance and timeliness of source material with reference to the specific research concerns arising from individual applications. © Austrian Red Cross/ACCORD An electronic version of this report is available on www.ecoi.net. Austrian Red Cross/ACCORD Wiedner Hauptstraße 32 A- 1040 Vienna, Austria Phone: +43 1 58 900 – 582 E-Mail: [email protected] Web: http://www.redcross.at/accord This report was commissioned by the United Nations High Commissioner for Refugees (UNHCR), Division of International Protection. UNHCR is not responsible for, nor does it endorse, its content. TABLE OF CONTENTS List of abbreviations ........................................................................................................................ 4 1 Background information ......................................................................................................... 6 1.1 Geographical information .................................................................................................... 6 1.1.1 Map of Ethiopia ........................................................................................................... -

The Impacts of Microcredit: Evidence from Ethiopia†
American Economic Journal: Applied Economics 2015, 7(1): 54–89 http://dx.doi.org/10.1257/app.20130475 The Impacts of Microcredit: Evidence from Ethiopia† By Alessandro Tarozzi, Jaikishan Desai, and Kristin Johnson* We use data from a randomized controlled trial conducted in 2003–2006 in rural Amhara and Oromiya Ethiopia to study the impacts of increasing access to microfinance( on a) number of socioeconomic outcomes, including income from agriculture, animal husbandry, nonfarm self-employment, labor supply, schooling and indicators of women’s empowerment. We document that despite sub- stantial increases in borrowing in areas assigned to treatment the null of no impact cannot be rejected for a large majority of outcomes. JEL G21, I20, J13, J16, O13, O16, O18 ( ) eginning in the 1970s, with the birth of the Grameen Bank in Bangladesh, Bmicrocredit has played a prominent role among development initiatives. Many proponents claim that microfinance has had enormously positive effects among borrowers. However, the rigorous evaluation of such claims of success has been complicated by the endogeneity of program placement and client selection, both common obstacles in program evaluations. Microfinance institutions MFIs typi- ( ) cally choose to locate in areas predicted to be profitable, and or where large impacts / are expected. In addition, individuals who seek out loans in areas served by MFIs and that are willing and able to form joint-liability borrowing groups a model often ( preferred by MFIs are likely different from others who do not along a number of ) observable and unobservable factors. Until recently, the results of most evaluations could not be interpreted as conclusively causal because of the lack of an appropriate control group see Brau and Woller 2004 and Armendáriz de Aghion and Morduch ( 2005 for comprehensive early surveys . -

OROMIA REGION - Regional 3W Map 07 December 2010
OROMIA REGION - Regional 3W Map 07 December 2010 CRS I SC-UK V Legend W Amhara S d S Farm Africa R S i E R C Benishangul R A V E CRS CARE MfM C GOAL C P n R W S o i I International Boundary SC-Denmark A , t Gumuz Afar C L c C P K Action Aid C A CARE Welthungerhilfe A CRS S U I M - I ! O C C IMC S S G S a CARE A WVE S Regional Boundary , SC-Denmark R R c m i SC-Denmark Dera C C a S r R f u u u c i C U E A Action Aid t r S m r n u f GOAL e R m R a r A m E IMC r i E C A b a I L L a R R Zonal Boundary K CARE ! f F o C Action Aid H s A a Hidabu Abote m r ! A A g a A r J x a a e A a C r Christian Aid C O d a C d r m O a r b O IMC Action Aid i W a M CA RE o J e I a G F G G G u e ! g L t n b r b e i a i i n i J o E S m u D a d Farm Africa Gerar Jarso R CARE Woreda Boundary E IMC u e p Kuyu E a ! Kiltu Kara m A i n ! o R ! S R C r ! a L Abay Chomen B Debre Libanos o ! Abuna G/Beret A M m M e A H e l o C en a m ks e ina r u Yaya Gulele Abichuna Gne'a C Ch Abe Dongoro ! a h s ! e i t c r a g f a u Nejo t IMC a ! r CRS No Data /No Intervention g E J ! W ! l a x Kombolcha o e R o ! d V e O b B i o ! ! s Guduru G J ib it Goro Gutu ! a r m b a Gudetu Kondole FHI Ke W a Mercy Corps b a s B u i ! r B Boji Dirmeji o t a Bila Seyo e i ! a r Jimma Genete l J d K L e g t s l Jeldu u E a ! d D ! u F Haro Maya e Guto Gida b M S u A m A o e B e M l R i d ! Boji Chekorsa Jimma Rare S ! D CARE GOAL CRS b a B b u A o Aleltu l e ! Kurfa Chele A g I O u f e e y G i u a S Mieso r Agriculture & Livestock i C r s t ! T a Mida KegnA a M Lalo Asabi im r K a ! ! G S Gobu -
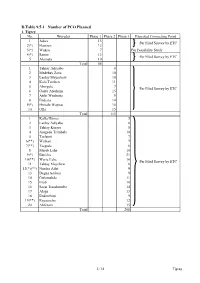
D.Table 9.5-1 Number of PCO Planned 1
D.Table 9.5-1 Number of PCO Planned 1. Tigrey No. Woredas Phase 1 Phase 2 Phase 3 Expected Connecting Point 1 Adwa 13 Per Filed Survey by ETC 2(*) Hawzen 12 3(*) Wukro 7 Per Feasibility Study 4(*) Samre 13 Per Filed Survey by ETC 5 Alamata 10 Total 55 1 Tahtay Adiyabo 8 2 Medebay Zana 10 3 Laelay Mayechew 10 4 Kola Temben 11 5 Abergele 7 Per Filed Survey by ETC 6 Ganta Afeshum 15 7 Atsbi Wenberta 9 8 Enderta 14 9(*) Hintalo Wajirat 16 10 Ofla 15 Total 115 1 Kafta Humer 5 2 Laelay Adiyabo 8 3 Tahtay Koraro 8 4 Asegede Tsimbela 10 5 Tselemti 7 6(**) Welkait 7 7(**) Tsegede 6 8 Mereb Lehe 10 9(*) Enticho 21 10(**) Werie Lehe 16 Per Filed Survey by ETC 11 Tahtay Maychew 8 12(*)(**) Naeder Adet 9 13 Degua temben 9 14 Gulomahda 11 15 Erob 10 16 Saesi Tsaedaemba 14 17 Alage 13 18 Endmehoni 9 19(**) Rayaazebo 12 20 Ahferom 15 Total 208 1/14 Tigrey D.Table 9.5-1 Number of PCO Planned 2. Affar No. Woredas Phase 1 Phase 2 Phase 3 Expected Connecting Point 1 Ayisaita 3 2 Dubti 5 Per Filed Survey by ETC 3 Chifra 2 Total 10 1(*) Mile 1 2(*) Elidar 1 3 Koneba 4 4 Berahle 4 Per Filed Survey by ETC 5 Amibara 5 6 Gewane 1 7 Ewa 1 8 Dewele 1 Total 18 1 Ere Bti 1 2 Abala 2 3 Megale 1 4 Dalul 4 5 Afdera 1 6 Awash Fentale 3 7 Dulecha 1 8 Bure Mudaytu 1 Per Filed Survey by ETC 9 Arboba Special Woreda 1 10 Aura 1 11 Teru 1 12 Yalo 1 13 Gulina 1 14 Telalak 1 15 Simurobi 1 Total 21 2/14 Affar D.Table 9.5-1 Number of PCO Planned 3. -

On the Ecology and Evolution of Microorganisms Associated with Fungus-Growing Termites
On the ecology and evolution of microorganisms associated with fungus-growing termites Anna A. Visser Propositions 1 Showing the occurrence of a certain organism in a mutualistic symbiosis does not prove a specific role of this organism for that mutualism, as is illustrated by Actinobacteria species occurring in fungus-growing termite nests. (this thesis) 2 Instead of playing a role as mutualistic symbiont, Pseudoxylaria behaves like a weed, competing for the fungus-comb substrate and forcing termites to do regular gardening lest it overgrows their Termitomyces monoculture. (this thesis) 3 Citing colleagues who are no longer active must be considered as an act of true altruism. 4 The overload of literature on recent ‘discoveries’ blinds us from old literature, causing researchers to neglect what was already known and possibly duplicate investigations. 5 Keys to evolution of knowledge lie in recognizing the truth of one’s intuition, and extending the limits of one’s imagination. 6 The presumed creative superiority of left-handed people (Newland 1981), said to be the result of more communication between both sides of the brain, might rather be the result of lifelong selection on finding creative solutions to survive in a right-hand biased environment. 7 An understanding of ‘why good ideas usually come by the time time is running out and how to manage this’, would greatly improve people’s intellectual output and the condition in which they perform. Propositions accompanying the thesis “On the ecology and evolution of microorganisms associated with fungus-growing termites” Anna A. Visser Wageningen, 15th June 2011 Reference Newland GA, 1981.