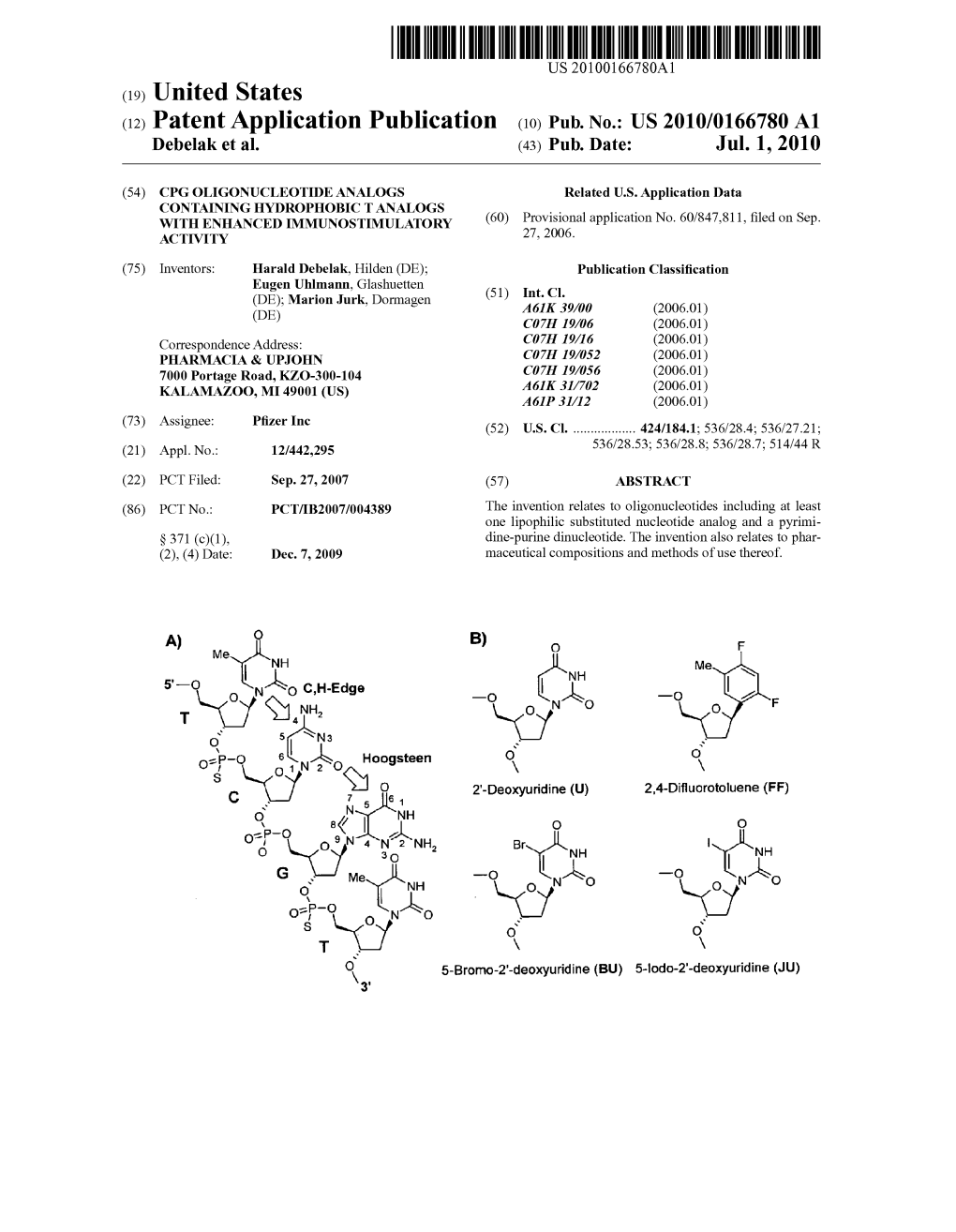
(12) Patent Application Publication (10) Pub. No.: US 2010/0166780 A1 Debelak Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Metabolomics Investigation Into the Effects of HIV Protease Inhibitors On
Molecular BioSystems View Article Online PAPER View Journal | View Issue A metabolomics investigation into the effects of HIV protease inhibitors on HPV16 E6 expressing Cite this: Mol. BioSyst., 2014, 10,398 cervical carcinoma cells† Dong-Hyun Kim,‡§a J. William Allwood,§¶a Rowan E. Moore,b Emma Marsden-Edwards,8b Warwick B. Dunn,¶a Yun Xu,a Lynne Hampson,c Ian N. Hampsonc and Royston Goodacre*ad Recently, it has been reported that anti-viral drugs, such as indinavir and lopinavir (originally targeted for HIV), also inhibit E6-mediated proteasomal degradation of mutant p53 in E6-transfected C33A cells. In order to understand more about the mode-of-action(s) of these drugs the metabolome of HPV16 E6 expressing cervical carcinoma cell lines was investigated using mass spectrometry (MS)-based metabolic profiling. The metabolite profiling of C33A parent and E6-transfected cells exposed to these two anti- viral drugs was performed by ultra performance liquid chromatography (UPLC)-MS and gas Creative Commons Attribution 3.0 Unported Licence. chromatography (GC)-time of flight (TOF)-MS. Using a combination of univariate and multivariate Received 23rd September 2013, analyses, these metabolic profiles were investigated for analytical and biological reproducibility and to Accepted 2nd January 2014 discover key metabolite differences elicited during anti-viral drug challenge. This approach revealed DOI: 10.1039/c3mb70423h both distinct and common effects of these two drugs on the metabolome of two different cell lines. Finally, intracellular drug levels were quantified, which suggested in the case of lopinavir that increased www.rsc.org/molecularbiosystems activity of membrane transporters may contribute to the drug sensitivity of HPV infected cells. -

Vidarabine Phosphate (BANM, USAN, Rinnm) Stability
912 Antivirals Pharmacopoeias. In US. ◊ Reviews. Pharmacopoeias. In US. USP 31 (Valganciclovir Hydrochloride). A white to off-white 1. Freeman RB. Valganciclovir: oral prevention and treatment of USP 31 (Vidarabine). A white to off-white powder. Very slightly powder. Freely soluble in alcohol; practically insoluble in ace- cytomegalovirus in the immunocompromised host. Expert Opin soluble in water; slightly soluble in dimethylformamide. Store in tone or in ethyl acetate; slightly soluble in hexane; very soluble Pharmacother 2004; 5: 2007–16. airtight containers. in isopropyl alcohol. Store in airtight containers at a temperature 2. Cvetković RS, Wellington K. Valganciclovir: a review of its use in the management of CMV infection and disease in immuno- of 25°, excursions permitted between 15° and 30°. compromised patients. Drugs 2005; 65: 859–78. Vidarabine Phosphate (BANM, USAN, rINNM) Stability. References. Administration in renal impairment. Doses of oral valgan- Ara-AMP; Arabinosyladenine Monophosphate; CI-808; Fosfato 1. Anaizi NH, et al. Stability of valganciclovir in an extemporane- ciclovir should be reduced in renal impairment according to cre- de vidarabina; Vidarabine 5′-Monophosphate; Vidarabine, Phos- ously compounded oral liquid. Am J Health-Syst Pharm 2002; atinine clearance (CC). Licensed product information recom- phate de; Vidarabini Phosphas. 9-β-D-Arabinofuranosyladenine 59: 1267–70. mends the following doses: 5′-(dihydrogen phosphate). 2. Henkin CC, et al. Stability of valganciclovir in extemporaneous- • CC 40 to 59 mL/minute: 450 mg twice daily for induction and Видарабина Фосфат ly compounded liquid formulations. Am J Health-Syst Pharm 2003; 60: 687–90. 450 mg daily for maintenance or prevention C10H14N5O7P = 347.2. -

WO 2011/103516 A2 25 August 2011 (25.08.2011) PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau I (10) International Publication Number (43) International Publication Date WO 2011/103516 A2 25 August 2011 (25.08.2011) PCT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/536 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) Number: International Application CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US201 1/025558 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 18 February 20 11 (18.02.201 1) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/305,862 18 February 2010 (18.02.2010) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): THE GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, TRUSTEES OF PRINCETON UNIVERSITY ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, [US/US]; P.O. -

(12) Patent Application Publication (10) Pub. No.: US 2002/0177609 A1 Swindell Et Al
US 2002O177609A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0177609 A1 Swindell et al. (43) Pub. Date: Nov. 28, 2002 (54) FATTY ALCOHOL DRUG CONJUGATES Related U.S. Application Data (60) Provisional application No. 60/278,457, filed on Mar. (76) Inventors: Charles S. Swindell, Merion, PA (US); 23, 2001. Glenn J. Fegley, Eagleville, PA (US) Publication Classification Correspondence Address: (51) Int. Cl." ..................... A61K 31/4453; A61K 31/40; Edward R. Gates, Esq. A61K 31/15 Chantal Morgan D'Apuzzo (52) U.S. Cl. ......................... 514/329; 514/426; 514/640; Wolf, Greenfield & Sacks, P.C. 546/244; 548/558; 564/256 600 Atlantic Ave Boston, MA 02210 (US) (57) ABSTRACT The invention provides conjugates of fatty alcohols and (21)21) AppAppl. No.: 10/107,537/107, p harmaceutical agents9. useful in treating9. cancer, Viruses, psychiatric disorders. Compositions, pharmaceutical prepa rations, and methods of preparation of the fatty alcohols 22) Filled: Mar. 25, 2002 p harmaceutical agent9. coniugatesJug are pprovided. US 2002/0177609 A1 Nov. 28, 2002 FATTY ALCOHOL DRUG CONJUGATES was observed (for an adenosine receptor agonist), and it was postulated that the pendant lipid molecule interacted with RELATED APPLICATION the phospholipid membrane to act as a distal anchor for the 0001. This application claims priority under 35 U.S.C. S receptor ligand in the membrane micro environment of the 119 (e) from U.S. provisional patent application Serial No. receptor. This increase in potency, however, was not 60/278,457, filed on Mar. 23, 2001, entitled Fatty Alcohol observed when the same lipid derivatives of adenosine Drug Conjugates. -

A Process for the Preparation of Fludarabine Phosphate from 2-Fluoroadenine
Europäisches Patentamt *EP001464708A1* (19) European Patent Office Office européen des brevets (11) EP 1 464 708 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C12P 19/32, C12P 19/40, 06.10.2004 Bulletin 2004/41 C07H 19/16, C07H 19/20 (21) Application number: 03007679.8 (22) Date of filing: 03.04.2003 (84) Designated Contracting States: • Petrucciani, Luigi AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 21100 Varese (IT) HU IE IT LI LU MC NL PT RO SE SI SK TR • Colombo, Paolo Designated Extension States: 21100 Varese (IT) AL LT LV MK • Caprioli, Giovanni 21100 Varese (IT) (71) Applicant: Pro. Bio. Sint. S.p.A. 26900 Lodi (IT) (74) Representative: Minoja, Fabrizio, Dr. Bianchetti Bracco Minoja S.r.l. (72) Inventors: Via Plinio, 63 • Farina, Paolo 20129 Milano (IT) 26900 Lodi (IT) (54) A process for the preparation of fludarabine phosphate from 2-fluoroadenine (57) The invention provides a process for the prep- EBA cell paste, to yield fludarabine. Fludarabine is then aration of fludarabine phosphate from 2-fluoroadenine treated with acetic anhydride and the resulting acetyl- and 9-β-D-arabinofuranosyl-uracil using Enterobacter derivative is crystallised and hydrolysed to fludarabine. aerogenes (EBA). Phosphorylation and crystallisation afford fludarabine 2-Fluoroadenine is reacted with 9-β-D-arabinosyl- phosphate. uracile in a water solution at pH = 7 in the presence of EP 1 464 708 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 464 708 A1 Description FIELD OF THE INVENTION 5 [0001] The present invention relates to a process for the preparation of fludarabine phosphate (I), in particular to a process for the preparation of fludarabine phosphate from 2-fluoroadenine and 9-β-D-arabinofuranosyluracil using Enterobacter aerogenes. -

Monophosphate in Patients with Chronic Hepatitis B
422 Gut 1995; 36: 422-426 Adenine arabinoside 5'-monophosphate in patients with chronic hepatitis B: comparison of the efficacy in patients with high and low viral Gut: first published as 10.1136/gut.36.3.422 on 1 March 1995. Downloaded from replication P Marcellin, M Pouteau, M A Loriot, N Boyer, F Degos, P Cales, L Bettan, Y Bacq, H Coppere, J Didier Grange, P H Bernard, C Degott, S Erlinger, J-P Benhamou Abstract Differences in the populations studied could This study compared the response to account for these discrepancies. In a previous adenine arabinoside 5'-monophosphate study, we found that anti-HIV negative homo- (ARA AMP) in 60 patients with chronic sexuals with chronic hepatitis B seemed to Service d'Hepatologie hepatitis B according to the pretreatment respond as frequently as heterosexuals to ARA and INSERM U24, serum hepatitis B virus DNA concentra- AMP treatment.3 In the same study, we found H6pital Beaujon, tion. The level ofhepatitis B virus replica- retrospectively that a low level of hepatitis B Clichy, France P Marcellin tion was defined as low (30 patients) or virus replication, as reflected by low serum M Pouteau high (30 patients) when serum hepatitis B hepatitis B virus DNA concentrations, was a M A Loriot virus DNA concentration was below or factor for predicting response to ARA AMP. N Boyer F Degos above 100 pg/ml, respectively. Patients The aims of this study were to assess, in S Erlinger received a 28 day course ofARA AMP and patients with chronic hepatitis B: (a) the J-P Benhamou a second course of ARA AMP was given response to ARA AMP according to the pre- to with treatment serum Service d'Hepato- six months later patients persis- concentration of hepatitis B Gastroent6rologie, tent hepatitis B virus replication. -

WO 2010/111485 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 30 September 2010 (30.09.2010) WO 2010/111485 Al (51) International Patent Classification: (74) Agent: LANDRUM, Charles, P.; Fulbright & Jaworski C12N 15/11 7 (2010.01) A61P 31/04 (2006.01) L.L.P., 600 Congress Ave., Suite 2400, Austin, TX 78701 A61K 38/06 (2006.01) A61P 31/10 (2006.01) (US). A61K 45/06 (2006.01) A61P 31/12 (2006.01) (81) Designated States (unless otherwise indicated, for every C07K 14/705 (2006.01) A61P 37/04 (2006.01) kind of national protection available): AE, AG, AL, AM, (21) International Application Number: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, PCT/US2010/028658 CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 25 March 2010 (25.03.2010) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (25) Filing Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (30) Priority Data: TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. 61/163,1 37 25 March 2009 (25.03.2009) US (84) Designated States (unless otherwise indicated, for every 61/179,246 18 May 2009 (18.05.2009) US kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): THE GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, BOARD OF REGENTS OF THE UNIVERSITY OF ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TEXAS SYSTEM [US/US]; 201 West 7th St., Austin, TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, TX 78701 (US). -

Universidade Estadual De Feira De Santana Dna E Rna
UNIVERSIDADE ESTADUAL DE FEIRA DE SANTANA PROGRAMA DE PÓS-GRADUAÇÃO EM BIOTECNOLOGIA BRUNO SILVA ANDRADE DNA E RNA POLIMERASES DO PLASMÍDEO MITOCONDRIAL DE MONILIOPHTHORA PERNICIOSA (STAHEL) AIME & PHILLIPS-MORA: CARACTERIZAÇÃO, EXPRESSÃO, ESTUDO DE INIBIDORES E FILOGENIA MOLECULAR Feira de Santana, BA 2011 2 BRUNO SILVA ANDRADE DNA E RNA POLIMERASES DO PLASMÍDEO MITOCONDRIAL DE MONILIOPHTHORA PERNICIOSA (STAHEL) AIME & PHILLIPS-MORA: CARACTERIZAÇÃO, EXPRESSÃO, ESTUDO DE INIBIDORES E FILOGENIA MOLECULAR Tese apresentada ao Programa de Pós-graduação em Biotecnologia, da Universidade Estadual de Feira de Santana como requisito parcial para obtenção do título de Doutor em Biotecnologia. Orientador: Prof. Dr. Aristóteles Góes Neto Feira de Santana, BA 2011 3 Dedico esta tese a minha mãe, Léa Maria S. Andrade. 4 AGRADECIMENTOS Agradeço especialmente ao meu amigo e orientador, Dr. Aristóteles Góes Neto , pelo apoio e acompanhamento dado para realização deste trabalho e principalmente por me tornar uma pessoa mais responsável e independente cientificamente, deixando-me construir minhas próprias idéias. À Paloma Araújo Bahia , pelo apoio emocional e afetivo, durante todos esses anos, aturando os meus momentos de “autismo” no período em estive escrevendo esta tese. Ao meu amigo Wagner Soares (UESB), pelas opiniões e sugestões de trabalho, pela parceria em projetos e pelos bons momentos de gargalhadas que demos durante todos esses anos. Agradeço a todos os professores e alunos do PPGBiotec-UEFS/Fiocruz, que conviveram comigo durante esse período, contribuindo de alguma maneira para a o meu trabalho. Ao secretário Helton Carneiro , pela amizade e competência em atender às solicitações diversas feitas durante a execução deste trabalho. À minha amiga Catiane Souza , pelo apoio de sempre, pelas ótimas discussões de trabalho, pela ótima companhia em viagens para congressos e cursos, pelas boas risadas que damos para descontrair. -

Lamivudine Treatment for Chronic Hepatitis B
LAMIVUDINE TREATMENT FOR CHRONIC HEPATITIS B P. Honkoop ISBN 90-9011356-8 All rights reserved. No part of this thesis may be reproduced, stored in a retrieval system of any nature, or transmitted in any form by any means, electronic, mechanical, photocopying, recording or otherwise, included a complete or partial transcription, without the prior pennission of the author. W Print: Offse.drui<kerij Ridderprin. B.Y" Ridderkerk LAMIVUDINE TREATMENT FOR CHRONIC HEPATITIS B LAMIVUDINE BEHANDELING VAN CHRONISCHE HEPATITIS B PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Erasmus Universiteit Rotterdam op gezag van de rector magnificus Prof. dr P. W.C. Akkermans M.A. en volgens besluit van het college voor promoties. De openbare verdediging zal plaatsvinden op woensdag 22 april 1998 om llAS uur door Pieter Honkoop geboren te Kampen Promotie commissie Promotor: Prof. dr S.W. Schalm Promotie commissie: Prof. dr W.J. Mooi Prof. dr H.R. Scholte Prof. dr AD.M.E. Osterhaus Co-promotor: Dr R.A. de Man The clinical studies on lamivudine were supported by a grant from Glaxo-\Vellcome. This study was performed at the department of Hepatogastroenterology of the Erasmus University Hospital, Dijkzigt, Rotterdam, The Netherlands. Financial support for this thesis was kindly given by G1axo-Wellcome, Byk, Janssen-Cilag, Schering-Plough, Smith Kline Beecham, MSD, Boehringer Mannheim, Roche, Astra, Yamanouchi and Zambon. "Gee! OilS heden ons dagelijks brood" Mattheus 6: J J Aan Willlla en Pieler jr. Aan mijn moeder Tel' nagedachtenis aan mijn vader -

― D13 - 1 ― 医学中央雑誌刊行会・医学用語シソーラス 第9版( 2019) カテゴリー別リスト
医学中央雑誌刊行会・医学用語シソーラス 第9版( 2019) カテゴリー別リスト Nucleic Acids, Nucleotides, and Nucleosides D13+ Antisense Elements D13-10+ # Antisense DNA D13-10-10+ # Antisense Oligodeoxyribonucleotides D13-10-10-10+ # * Mipomersen D13-10-10-10-10 # Trecovirsen D13-10-10-10-20 # Antisense Oligonucleotides D13-10-20+ # Antisense Oligodeoxyribonucleotides D13-10-20-10+ # * Mipomersen D13-10-20-10-10 # Trecovirsen D13-10-20-10-20 # Antisense Oligoribonucleotides D13-10-20-20+ # * Nusinersen D13-10-20-20-10 # * Eteplirsen D13-10-20-30 # Fomivirsen D13-10-20-40 # Antisense RNA D13-10-30+ # Antisense Oligoribonucleotides D13-10-30-10+ # * Nusinersen D13-10-30-10-10 # MicroRNAs D13-10-30-20+ # * 循環MicroRNA D13-10-30-20-10 # siRNA D13-10-30-30+ # * Patisiran D13-10-30-30-10 # Nucleosides D13-20+ # Arabinonucleosides D13-20-10+ Arabinofuranosyluracil D13-20-10-10+ # Fialuridine D13-20-10-10-10 # Netivudine D13-20-10-10-20 # Sorivudine D13-20-10-10-30 # Clofarabine D13-20-10-20 # Cytarabine D13-20-10-30+ # Ancitabine D13-20-10-30-10 # Enocitabine D13-20-10-30-20 # Fiacitabine D13-20-10-30-30 # Nelarabine D13-20-10-40 # Sapacitabine D13-20-10-50 # Vidarabine D13-20-10-60+ # Fludarabine D13-20-10-60-10 # Deoxyribonucleosides D13-20-20+ Deoxyadenosines D13-20-20-10+ # Cladribine D13-20-20-10-10 # Dideoxyadenosine D13-20-20-10-20 # Puromycin Aminonucleoside D13-20-20-10-30 # Deoxycytidine D13-20-20-20+ # Apricitabine D13-20-20-20-10 # Bromodeoxycytidine D13-20-20-20-20 # Capecitabine D13-20-20-20-30 # Emtricitabine D13-20-20-20-40+ # * Elvitegravir-Cobicistat-Emtricitabine-Tenofovir -
(12) Patent Application Publication (10) Pub. No.: US 2002/0010208A1 Shashoua Et Al
US 2002001 0208A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0010208A1 Shashoua et al. (43) Pub. Date: Jan. 24, 2002 (54) DHA-PHARMACEUTICAL AGENT Related U.S. Application Data CONJUGATES OF TAXANES (63) Continuation of application No. 09/135,291, filed on (76) Inventors: Victor Shashoua, Brookline, MA (US); Aug. 17, 1998, now abandoned, which is a continu Charles Swindell, Merion, PA (US); ation of application No. 08/651,312, filed on May 22, Nigel Webb, Bryn Mawr, PA (US); 1996, now Pat. No. 5,795,909. Matthews Bradley, Layton, PA (US) Publication Classification Correspondence Address: Edward R. Gates, Esq. (51) Int. Cl." ............................................ A61K 31/337 Wolf, Greenfield & Sacks, P.C. (52) U.S. Cl. .............................................................. 514/449 600 Atlantic Avenue Boston, MA 02210 (US) (57) ABSTRACT The invention provides conjugates of cis-docosahexaenoic (21) Appl. No.: 09/846,838 acid and pharmaceutical agents useful in treating noncentral nervous System conditions. Methods for Selectively target 22) Filled: Mayy 1, 2001 ingg pharmaceuticalp agents9. to desired tissues are pprovided. Patent Application Publication Jan. 24, 2002 Sheet 1 of 14 US 2002/0010208A1 1 OO 5 O -5OO - 1 OO-9 -8 -7 -6 -5 -4 LOG-10 OF SAMPLE CONCENTRATION (MOLAR) CCRF-CEM-o- SR ----- RPM-8226----- K-562- - -A - - HL-60 (TB) -g- - MOLT4: ... O Fig. 1 1 OO 5 O -5OO -1 O O -8 -7 -6 -5 -4 -- 9 LOGo OF SAMPLE CONCENTRATION (MOLAR) A549/ATCC-o-NS326. NCEKVX 28. --Q-- NCI-H322M-...-a---Eidsf8::... NC-H522--O-- HOP-62---Fig. 2 a-- NC-H460.-------- Patent Application Publication Jan. -
Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs
Molecules 2016, 21, 75; doi:10.3390/molecules21010075 S1 of S110 Supplementary Materials: Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs Fei Mao 1, Wei Ni 1, Xiang Xu 1, Hui Wang 1, Jing Wang 1, Min Ji 1 and Jian Li * Table S1. Common names, indications, CAS Registry Numbers and molecular formulas of 6891 approved drugs. Common Name Indication CAS Number Oral Molecular Formula Abacavir Antiviral 136470-78-5 Y C14H18N6O Abafungin Antifungal 129639-79-8 C21H22N4OS Abamectin Component B1a Anthelminithic 65195-55-3 C48H72O14 Abamectin Component B1b Anthelminithic 65195-56-4 C47H70O14 Abanoquil Adrenergic 90402-40-7 C22H25N3O4 Abaperidone Antipsychotic 183849-43-6 C25H25FN2O5 Abecarnil Anxiolytic 111841-85-1 Y C24H24N2O4 Abiraterone Antineoplastic 154229-19-3 Y C24H31NO Abitesartan Antihypertensive 137882-98-5 C26H31N5O3 Ablukast Bronchodilator 96566-25-5 C28H34O8 Abunidazole Antifungal 91017-58-2 C15H19N3O4 Acadesine Cardiotonic 2627-69-2 Y C9H14N4O5 Acamprosate Alcohol Deterrant 77337-76-9 Y C5H11NO4S Acaprazine Nootropic 55485-20-6 Y C15H21Cl2N3O Acarbose Antidiabetic 56180-94-0 Y C25H43NO18 Acebrochol Steroid 514-50-1 C29H48Br2O2 Acebutolol Antihypertensive 37517-30-9 Y C18H28N2O4 Acecainide Antiarrhythmic 32795-44-1 Y C15H23N3O2 Acecarbromal Sedative 77-66-7 Y C9H15BrN2O3 Aceclidine Cholinergic 827-61-2 C9H15NO2 Aceclofenac Antiinflammatory 89796-99-6 Y C16H13Cl2NO4 Acedapsone Antibiotic 77-46-3 C16H16N2O4S Acediasulfone Sodium Antibiotic 80-03-5 C14H14N2O4S Acedoben Nootropic 556-08-1 C9H9NO3 Acefluranol Steroid