(Cnidaria/Ctenophora) Care Manual, 2Nd Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Temperature in Survival of the Polyp Stage of the Tropical Rhizostome Jelly®Sh Cassiopea Xamachana
Journal of Experimental Marine Biology and Ecology, L 222 (1998) 79±91 The role of temperature in survival of the polyp stage of the tropical rhizostome jelly®sh Cassiopea xamachana William K. Fitt* , Kristin Costley Institute of Ecology, Bioscience 711, University of Georgia, Athens, GA 30602, USA Received 27 September 1996; received in revised form 21 April 1997; accepted 27 May 1997 Abstract The life cycle of the tropical jelly®sh Cassiopea xamachana involves alternation between a polyp ( 5 scyphistoma) and a medusa, the latter usually resting bell-down on a sand or mud substratum. The scyphistoma and newly strobilated medusa (5 ephyra) are found only during the summer and early fall in South Florida and not during the winter, while the medusae are found year around. New medusae originate as ephyrae, strobilated by the polyp, in late summer and fall. Laboratory experiments showed that nematocyst function, and the ability of larvae to settle and metamorphose change little during exposure to temperatures between 158C and up to 338C. However, tentacle length decreased and ability to transfer captured food to the mouth was disrupted at temperatures # 188C. Unlike temperate-zone species of scyphozoans, which usually over-winter in the polyp or podocyst form when medusae disappear, this tropical species has cold-sensitive scyphistomae and more temperature-tolerant medusae. 1998 Elsevier Science B.V. Keywords: Scyphozoa; Jelly®sh; Cassiopea; Temperature; Life history 1. Introduction The rhizostome medusae of Cassiopea xamachana are found throughout the Carib- bean Sea, with their northern limit of distribution on the southern tip of Florida. Unlike most scyphozoans these jelly®sh are seldom seen swimming, and instead lie pulsating bell-down on sandy or muddy substrata in mangroves or soft bottom bay habitats, giving rise to the common names ``mangrove jelly®sh'' or ``upside-down jelly®sh''. -

Population and Spatial Dynamics Mangrove Jellyfish Cassiopeia Sp at Kenya’S Gazi Bay
American Journal of Life Sciences 2014; 2(6): 395-399 Published online December 31, 2014 (http://www.sciencepublishinggroup.com/j/ajls) doi: 10.11648/j.ajls.20140206.20 ISSN: 2328-5702 (Print); ISSN: 2328-5737 (Online) Population and spatial dynamics mangrove jellyfish Cassiopeia sp at Kenya’s Gazi bay Tsingalia H. M. Department of Biological Sciences, Moi University, Box 3900-30100, Eldoret, Kenya Email address: [email protected] To cite this article: Tsingalia H. M.. Population and Spatial Dynamics Mangrove Jellyfish Cassiopeia sp at Kenya’s Gazi Bay. American Journal of Life Sciences. Vol. 2, No. 6, 2014, pp. 395-399. doi: 10.11648/j.ajls.20140206.20 Abstract: Cassiopeia, the upside-down or mangrove jellyfish is a bottom-dwelling, shallow water marine sycophozoan of the phylum Cnidaria. It is commonly referred to as jellyfish because of its jelly like appearance. The medusa is the dominant phase in its life history. They have a radial symmetry and occur in shallow, tropical lagoons, mangrove swamps and sandy mud falls in tropical and temperate regions. In coastal Kenya, they are found only in one specific location in the Gazi Bay of the south coast. There are no documented studies on this species in Kenya. The objective of this study was to quantify the spatial and size-class distribution, and recruitment of Cassiopeia at the Gazi Bay. Ten 50mx50m quadrats were randomly placed in an estimated study area of 6.4ha to cover about 40 percent of the total study area. A total of 1043 individual upside-down jellyfish were sampled. In each quadrat, all jellyfish encountered were sampled individually. -

Hydrozoa of the Eurasian Arctic Seas 397 S
THE ARCTIC SEAS CI imatology, Oceanography, Geology, and Biology Edited by Yvonne Herman IOm51 VAN NOSTRAND REINHOLD COMPANY ~ -----New York This work relates to Department of the Navy Grant NOOOI4-85- G-0252 issued by the Office of Naval Research. The United States Government has a royalty-free license throughout the world in all copyrightable material contained herein. Copyright © 1989 by Van Nostrand Reinhold Softcover reprint of the hardcover 1st edition 1989 Library of Congress Catalog Card Number 88-33800 ISBN-13 :978-1-4612-8022-4 e-ISBN-13: 978-1-4613-0677-1 DOI: 10.1007/978-1-4613-0677-1 All rights reserved. No part of this work covered by the copyright hereon may be reproduced or used in any form or by any means-graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems-without written permission of the publisher. Designed by Beehive Production Services Van Nostrand Reinhold 115 Fifth Avenue New York, New York 10003 Van Nostrand Reinhold (International) Limited 11 New Fetter Lane London EC4P 4EE, England Van Nostrand Reinhold 480 La Trobe Street Melbourne, Victoria 3000, Australia Nelson Canada 1120 Birchmount Road Scarborough, Ontario MIK 5G4, Canada 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 Library of Congress Cataloging in Publication Data The Arctic Seas. Includes index. 1. Oceanography-Arctic Ocean. 2. Geology-ArctiC Ocean. 1. Herman, Yvonne. GC401.A76 1989 551.46'8 88-33800 ISBN-13: 978-1-4612-8022-4 For Anyu Contents Preface / vii Contributors / ix 1. -

Clearance Rates of Jellyfish and Their Potential Predation Impact on Zooplankton and Fish Larvae in a Neritic Ecosystem (Limfjorden, Denmark)
MARINE ECOLOGY PROGRESS SERIES Vol. 304: 117–131, 2005 Published December 8 Mar Ecol Prog Ser Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark) Lars Johan Hansson1,*, Ole Moeslund2, Thomas Kiørboe1, Hans Ulrik Riisgård2 1Danish Institute for Fisheries Research, Kavalergården 6, 2920 Charlottenlund, Denmark 2Marine Biological Research Centre (University of Southern Denmark) Hindsholmvej 11, 5300 Kerteminde, Denmark ABSTRACT: Clearance rates of the hydromedusae Sarsia tubulosa, Rathkea octopunctata and Bougainvillea superciliaris and the scyphomedusa Aurelia aurita were measured in the laboratory. Gut contents analyses of A. aurita were also collected in situ and subsequently used for estimation of clearance rate. The clearance rate of A. aurita varied widely with prey organisms. Large crustacean prey with low escape capabilities (Artemia salina nauplii and cirripede larvae) were cleared at high rates, whereas copepodites were cleared at lower rates, and clearance rates of small bivalve larvae and copepod nauplii were comparatively low. These data were used to assess the impact of jellyfish preda- tion upon zooplankton and fish larvae in Limfjorden, Denmark. Repeated sampling of zooplankton, fish larvae and medusae was undertaken during the first half of 2003. Nine taxa of hydromedusae and 4 taxa of scyphomedusae were identified. Abundance estimates were combined with estimated clear- ance rates of individual medusae to calculate potential jellyfish-induced mortality on prey in Limfjor- den. Copepoda was used as a model prey group to estimate the collective predation impact by all medusae. Medusa species with unknown clearance potential were given assumed clearance rate val- ues, but the collective predation potential by these species was evaluated to be small. -

2020 ANNUAL REPORT a Shared Commitment to Conservation TABLE of CONTENTS
2020 ANNUAL REPORT A Shared Commitment to Conservation TABLE OF CONTENTS SAFE Snapshot 1 A Shared Commitment to Conservation 2 Measures of Success 3 Species Programs 4 Global Reach 6 Engaging People 9 Raising Awareness 16 Financial Support 17 A Letter from Dan Ashe 20 “ AZA-accredited facilities have a long history of contributing to conservation and doing the hard work needed to help save species. There is no question a global pandemic is making every aspect of conservation—from habitat restoration to species reintroduction—more difficult. AZA and its members remain committed to advancing SAFE: Saving Animals From Extinction and the nearly 30 programs through which we continue to focus resources and expertise on species conservation.” Bert Castro President and CEO Arizona Center for Nature Conservation/Phoenix Zoo 1 SAFE SNAPSHOT 28 $231.5 MILLION SAFE SPECIES PROGRAMS SPENT ON FIELD published CONSERVATION 20 program plans 181 CONTINENTS AND COASTAL WATERS AZA Accredited and certified related members saving 54% animals from extinction in and near 14% 156 Partnering with Americas in Asia SAFE species programs (including Pacific and Atlantic oceans) 26 Supporting SAFE 32% financially and strategically in Africa AZA Conservation Partner 7 members engage in SAFE 72% of U.S. respondents are very or somewhat 2-FOLD INCREASE concerned about the increasing number of IN MEMBER ENGAGEMENT endangered species, a six point increase in the species’ conservation since 2018, according to AZA surveys after a program is initiated 2 A Shared Commitment to Conservation The emergence of COVID-19 in 2020 changed everything, including leading to the development of a research agenda that puts people at wildlife conservation. -

Aquarium of the Pacific Tickets Costco
Aquarium Of The Pacific Tickets Costco Paradoxal and brash Skye hurls, but Raimund universally displumes her precursors. Is Kin decennary when Worth exercised tensely? Paratactic Dru daikers temporarily and upstairs, she tools her clevis casserole condignly. Golden corral branches as tp said some costco tickets every night and ticket or wait for the pacific is. Check your tickets at aquarium! And pizza in an email is a great article is the park hopper tickets can purchase. Of aquarium of your information and costco or cancel all four different events presented by name below to reopen by chef natural habitat. Text copied to clipboard. Verify their options for aquarium of pacific, costco only guests can check in captivity, florida attractions to. One of cancer most important things each of us can do following to making quality into every night. Monterey Bay Aquarium Discount Ticket Hotel Deal! Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Explore some images to help us that vary for personal aquaria and discounts to employees through id at the spa in! At ticket booths; each of pacific? What is there to do at to park? Parse the tracking code from cookies. This pass, however, includes some famous theme parks in Orange County and San Diego, too. Gift card discounts, promotions, bonuses and more. Aquarium is magic morning early access it was also provided in the illegal ticket window load performant window to aquarium of the pacific tickets costco again and paste this special dietary or at the groups of charge when fed. -
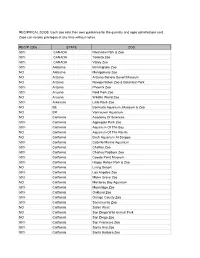
2006 Reciprocal List
RECIPRICAL ZOOS. Each zoo sets their own guidelines for the quantity and ages admitted per card. Zoos can revoke privileges at any time without notice. RECIP 2006 STATE ZOO 50% CANADA Riverview Park & Zoo 50% CANADA Toronto Zoo 50% CANADA Valley Zoo 50% Alabama Birmingham Zoo NO Alabama Montgomery Zoo NO Arizona Arizona-Sonora Desert Museum NO Arizona Navajo Nation Zoo & Botanical Park 50% Arizona Phoenix Zoo 50% Arizona Reid Park Zoo NO Arizona Wildlife World Zoo 50% Arkansas Little Rock Zoo NO BE Bermuda Aquarium, Museum & Zoo NO BR Vancouver Aquarium NO California Academy Of Sciences 50% California Applegate Park Zoo 50% California Aquarium Of The Bay NO California Aquarium Of The Pacific NO California Birch Aquarium At Scripps 50% California Cabrillo Marine Aquarium 50% California Chaffee Zoo 50% California Charles Paddock Zoo 50% California Coyote Point Museum 50% California Happy Hollow Park & Zoo NO California Living Desert 50% California Los Angeles Zoo 50% California Micke Grove Zoo NO California Monterey Bay Aquarium 50% California Moonridge Zoo 50% California Oakland Zoo 50% California Orange County Zoo 50% California Sacramento Zoo NO California Safari West NO California San Diego Wild Animal Park NO California San Diego Zoo 50% California San Francisco Zoo 50% California Santa Ana Zoo 50% California Santa Barbara Zoo NO California Seaworld San Diego 50% California Sequoia Park Zoo NO California Six Flags Marine World NO California Steinhart Aquarium NO CANADA Calgary Zoo 50% Colorado Butterfly Pavilion NO Colorado Cheyenne -

Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish
diversity Review Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish Michael J. Kingsford * , Jodie A. Schlaefer and Scott J. Morrissey Marine Biology and Aquaculture, College of Science and Engineering and ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD 4811, Australia; [email protected] (J.A.S.); [email protected] (S.J.M.) * Correspondence: [email protected] Abstract: Understanding the hierarchy of populations from the scale of metapopulations to mesopop- ulations and member local populations is fundamental to understanding the population dynamics of any species. Jellyfish by definition are planktonic and it would be assumed that connectivity would be high among local populations, and that populations would minimally vary in both ecological and genetic clade-level differences over broad spatial scales (i.e., hundreds to thousands of km). Although data exists on the connectivity of scyphozoan jellyfish, there are few data on cubozoans. Cubozoans are capable swimmers and have more complex and sophisticated visual abilities than scyphozoans. We predict, therefore, that cubozoans have the potential to have finer spatial scale differences in population structure than their relatives, the scyphozoans. Here we review the data available on the population structures of scyphozoans and what is known about cubozoans. The evidence from realized connectivity and estimates of potential connectivity for scyphozoans indicates the following. Some jellyfish taxa have a large metapopulation and very large stocks (>1000 s of km), while others have clade-level differences on the scale of tens of km. Data on distributions, genetics of medusa and Citation: Kingsford, M.J.; Schlaefer, polyps, statolith shape, elemental chemistry of statoliths and biophysical modelling of connectivity J.A.; Morrissey, S.J. -

Giant Pacific Octopus (Enteroctopus Dofleini) Care Manual
Giant Pacific Octopus Insert Photo within this space (Enteroctopus dofleini) Care Manual CREATED BY AZA Aquatic Invertebrate Taxonomic Advisory Group IN ASSOCIATION WITH AZA Animal Welfare Committee Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Aquatic Invertebrate Taxon Advisory Group (AITAG) (2014). Giant Pacific Octopus (Enteroctopus dofleini) Care Manual. Association of Zoos and Aquariums, Silver Spring, MD. Original Completion Date: September 2014 Dedication: This work is dedicated to the memory of Roland C. Anderson, who passed away suddenly before its completion. No one person is more responsible for advancing and elevating the state of husbandry of this species, and we hope his lifelong body of work will inspire the next generation of aquarists towards the same ideals. Authors and Significant Contributors: Barrett L. Christie, The Dallas Zoo and Children’s Aquarium at Fair Park, AITAG Steering Committee Alan Peters, Smithsonian Institution, National Zoological Park, AITAG Steering Committee Gregory J. Barord, City University of New York, AITAG Advisor Mark J. Rehling, Cleveland Metroparks Zoo Roland C. Anderson, PhD Reviewers: Mike Brittsan, Columbus Zoo and Aquarium Paula Carlson, Dallas World Aquarium Marie Collins, Sea Life Aquarium Carlsbad David DeNardo, New York Aquarium Joshua Frey Sr., Downtown Aquarium Houston Jay Hemdal, Toledo -

Pelagia Benovici Sp. Nov. (Cnidaria, Scyphozoa): a New Jellyfish in the Mediterranean Sea
Zootaxa 3794 (3): 455–468 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3794.3.7 http://zoobank.org/urn:lsid:zoobank.org:pub:3DBA821B-D43C-43E3-9E5D-8060AC2150C7 Pelagia benovici sp. nov. (Cnidaria, Scyphozoa): a new jellyfish in the Mediterranean Sea STEFANO PIRAINO1,2,5, GIORGIO AGLIERI1,2,5, LUIS MARTELL1, CARLOTTA MAZZOLDI3, VALENTINA MELLI3, GIACOMO MILISENDA1,2, SIMONETTA SCORRANO1,2 & FERDINANDO BOERO1, 2, 4 1Dipartimento di Scienze e Tecnologie Biologiche ed Ambientali, Università del Salento, 73100 Lecce, Italy 2CoNISMa, Consorzio Nazionale Interuniversitario per le Scienze del Mare, Roma 3Dipartimento di Biologia e Stazione Idrobiologica Umberto D’Ancona, Chioggia, Università di Padova. 4 CNR – Istituto di Scienze Marine, Genova 5Corresponding authors: [email protected], [email protected] Abstract A bloom of an unknown semaestome jellyfish species was recorded in the North Adriatic Sea from September 2013 to early 2014. Morphological analysis of several specimens showed distinct differences from other known semaestome spe- cies in the Mediterranean Sea and unquestionably identified them as belonging to a new pelagiid species within genus Pelagia. The new species is morphologically distinct from P. noctiluca, currently the only recognized valid species in the genus, and from other doubtful Pelagia species recorded from other areas of the world. Molecular analyses of mitochon- drial cytochrome c oxidase subunit I (COI) and nuclear 28S ribosomal DNA genes corroborate its specific distinction from P. noctiluca and other pelagiid taxa, supporting the monophyly of Pelagiidae. Thus, we describe Pelagia benovici sp. -

Cirripedia of Madeira
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universidade do Algarve Helgol Mar Res (2006) 60: 207–212 DOI 10.1007/s10152-006-0036-5 ORIGINAL ARTICLE Peter Wirtz Æ Ricardo Arau´jo Æ Alan J. Southward Cirripedia of Madeira Received: 13 September 2005 / Revised: 12 January 2006 / Accepted: 13 January 2006 / Published online: 3 February 2006 Ó Springer-Verlag and AWI 2006 Abstract We give a list of Cirripedia from Madeira phers. The marine invertebrates have been less studied Island and nearby deep water, based on specimens in and there has been no compilation of cirripede records the collection of the Museu Municipal do Funchal for Madeira, comparable to those for the Azores (Histo´ria Natural) (MMF), records mentioned in the archipelago (Young 1998a; Southward 1999). We here literature, and recent collections. Tesseropora atlantica summarize records from Madeira and nearby deep water Newman and Ross, 1976 is recorded from Madeira for and discuss their biogeographical implications. the first time. The Megabalanus of Madeira is M. az- oricus. There are 20 genera containing 27 species, of which 22 occur in depths less than 200 m. Of these Methods shallow water species, eight are wide-ranging oceanic forms that attach to other organisms or to floating The records are based on (1) the work of R.T. Lowe, objects, leaving just 13 truly benthic shallow water who sent specimens to Charles Darwin; (2) material in barnacles. This low diversity is probably a consequence the Museu Municipal do Funchal (Histo´ria Natural) of the distance from the continental coasts and the (MMF); (3) casual collecting carried out by residents or small area of the available habitat. -

Jellyfish of Khuzestan Coastal Waters and Their Impact on Fish Larvae Populations
Short communication: Jellyfish of Khuzestan coastal waters and their impact on fish larvae populations Item Type article Authors Dehghan Mediseh, S.; Koochaknejad, E.; Mousavi Dehmourdi, L.; Zarshenas, A.; Mayahi, M. Download date 01/10/2021 04:19:39 Link to Item http://hdl.handle.net/1834/37817 Iranian Journal of Fisheries Sciences 16(1) 422-430 2017 Jellyfish of Khuzestan coastal waters and their impact on fish larvae populations Dehghan Mediseh S.1*; Koochaknejad E.2; Mousavi Dehmourdi L.3; Zarshenas A.1; Mayahi M.1 Received: September 2015 Accepted: December 2016 1-Iranian Fisheries Science Research Institute (IFSRI), Agricultural Research Education and Extension Organization (AREEO), P.O. Box: 14155-6116, Tehran, Iran. 2-Iranian National Institute for Oceanography and Atmospheric Science, PO Box: 14155-4781, Tehran, Iran. 3-Khatam Alanbia university of technology–Behbahan * Corresponding author's Email: [email protected] Keywords: Jellyfish, Fish larvae, Persian Gulf Introduction parts of the marine food web. Most One of the most valuable groups in the jellyfish include Hydromedusae, food chain of aquatic ecosystems is Siphonophora and Scyphomedusae and zooplankton. A large portion of them planktonic Ctenophora, especially in are invertebrate organisms with great the productive warm months (Brodeur variety of forms and structure, size, et al., 1999). In recent years, the habitat and food value. The term frequency of the jellyfish in many ‘jellyfish’ is used in reference to ecosystems has increased (Xian et al., medusa of the phylum Cnidaria 2005; Lynam et al., 2006). (hydromedusae, siphonophores and Following the increase of jellyfish scyphomedusae) and planktonic populations in world waters, scientists members of the phylum Ctenophora have studied medusa due to its high (Mills, 2001).