Estimating the Range Shift and Harvesting Intensity of Junipers in Bhutan (Eastern Himalaya)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Analyses of Juniperus Species in Turkey and Their Relations with Other Juniperus Based on Cpdna Supervisor: Prof
MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY AYSUN DEMET GÜVENDİREN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY APRIL 2015 Approval of the thesis MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA submitted by AYSUN DEMET GÜVENDİREN in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Department of Biological Sciences, Middle East Technical University by, Prof. Dr. Gülbin Dural Ünver Dean, Graduate School of Natural and Applied Sciences Prof. Dr. Orhan Adalı Head of the Department, Biological Sciences Prof. Dr. Zeki Kaya Supervisor, Dept. of Biological Sciences METU Examining Committee Members Prof. Dr. Musa Doğan Dept. Biological Sciences, METU Prof. Dr. Zeki Kaya Dept. Biological Sciences, METU Prof.Dr. Hayri Duman Biology Dept., Gazi University Prof. Dr. İrfan Kandemir Biology Dept., Ankara University Assoc. Prof. Dr. Sertaç Önde Dept. Biological Sciences, METU Date: iii I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name : Aysun Demet GÜVENDİREN Signature : iv ABSTRACT MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA Güvendiren, Aysun Demet Ph.D., Department of Biological Sciences Supervisor: Prof. -

Morphology and Morphogenesis of the Seed Cones of the Cupressaceae - Part II Cupressoideae
1 2 Bull. CCP 4 (2): 51-78. (10.2015) A. Jagel & V.M. Dörken Morphology and morphogenesis of the seed cones of the Cupressaceae - part II Cupressoideae Summary The cone morphology of the Cupressoideae genera Calocedrus, Thuja, Thujopsis, Chamaecyparis, Fokienia, Platycladus, Microbiota, Tetraclinis, Cupressus and Juniperus are presented in young stages, at pollination time as well as at maturity. Typical cone diagrams were drawn for each genus. In contrast to the taxodiaceous Cupressaceae, in Cupressoideae outgrowths of the seed-scale do not exist; the seed scale is completely reduced to the ovules, inserted in the axil of the cone scale. The cone scale represents the bract scale and is not a bract- /seed scale complex as is often postulated. Especially within the strongly derived groups of the Cupressoideae an increased number of ovules and the appearance of more than one row of ovules occurs. The ovules in a row develop centripetally. Each row represents one of ascending accessory shoots. Within a cone the ovules develop from proximal to distal. Within the Cupressoideae a distinct tendency can be observed shifting the fertile zone in distal parts of the cone by reducing sterile elements. In some of the most derived taxa the ovules are no longer (only) inserted axillary, but (additionally) terminal at the end of the cone axis or they alternate to the terminal cone scales (Microbiota, Tetraclinis, Juniperus). Such non-axillary ovules could be regarded as derived from axillary ones (Microbiota) or they develop directly from the apical meristem and represent elements of a terminal short-shoot (Tetraclinis, Juniperus). -

Extract of Juniperus Indica Bertol Synergizes with Cisplatin to Inhibit
molecules Article Extract of Juniperus indica Bertol Synergizes with Cisplatin to Inhibit Oral Cancer Cell Growth via Repression of Cell Cycle Progression and Activation of the Caspase Cascade Xiao-Fan Huang 1,2, Kai-Fu Chang 1,2 , Shan-Chih Lee 3,4, Chia-Yu Li 5, Hung-Hsiu Liao 2, Ming-Chang Hsieh 2,6,* and Nu-Man Tsai 2,6,* 1 Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; [email protected] (X.-F.H.); [email protected] (K.-F.C.) 2 Department of Medical Laboratory and Biotechnology, Chung Shan Medical University, Taichung 40201, Taiwan; [email protected] 3 Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung 40201, Taiwan; [email protected] 4 Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung 40201, Taiwan 5 Department of Life and Death, Nanhua University, Chiayi 62249, Taiwan; [email protected] 6 Clinical Laboratory, Chung Shan Medical University Hospital, Taichung 40201, Taiwan * Correspondence: [email protected] (M.-C.H.); [email protected] (N.-M.T.); Tel.: +886-4-2473-0022 (ext. 12411) (N.-M.T.); Fax: +886-4-2324-8171 (N.-M.T.) Academic Editors: Halina Ekiert and Agnieszka Szopa Received: 21 May 2020; Accepted: 12 June 2020; Published: 13 June 2020 Abstract: Oral cancer—a type of head and neck cancer—is estimated to be the fifth most common cancer in Taiwan. However, efficacious therapies for oral cancer are still lacking due to drug resistance and recurrence. Consequently, the identification of new anticancer agents for clinical treatment is needed. -

Himalayan Aromatic Medicinal Plants: a Review of Their Ethnopharmacology, Volatile Phytochemistry, and Biological Activities
medicines Review Himalayan Aromatic Medicinal Plants: A Review of their Ethnopharmacology, Volatile Phytochemistry, and Biological Activities Rakesh K. Joshi 1, Prabodh Satyal 2 and Wiliam N. Setzer 2,* 1 Department of Education, Government of Uttrakhand, Nainital 263001, India; [email protected] 2 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-256-824-6519; Fax: +1-256-824-6349 Academic Editor: Lutfun Nahar Received: 24 December 2015; Accepted: 3 February 2016; Published: 19 February 2016 Abstract: Aromatic plants have played key roles in the lives of tribal peoples living in the Himalaya by providing products for both food and medicine. This review presents a summary of aromatic medicinal plants from the Indian Himalaya, Nepal, and Bhutan, focusing on plant species for which volatile compositions have been described. The review summarizes 116 aromatic plant species distributed over 26 families. Keywords: Jammu and Kashmir; Himachal Pradesh; Uttarakhand; Nepal; Sikkim; Bhutan; essential oils 1. Introduction The Himalya Center of Plant Diversity [1] is a narrow band of biodiversity lying on the southern margin of the Himalayas, the world’s highest mountain range with elevations exceeding 8000 m. The plant diversity of this region is defined by the monsoonal rains, up to 10,000 mm rainfall, concentrated in the summer, altitudinal zonation, consisting of tropical lowland rainforests, 100–1200 m asl, up to alpine meadows, 4800–5500 m asl. Hara and co-workers have estimated there to be around 6000 species of higher plants in Nepal, including 303 species endemic to Nepal and 1957 species restricted to the Himalayan range [2–4]. -
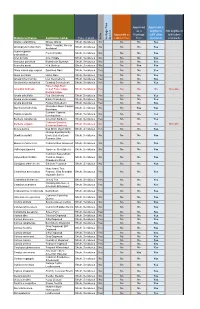
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Abiotic Factors and Yushania Influences on Abies Forest Composition in Taiwan
Taiwania, 59(3): 247‒261, 2014 DOI: 10.6165/tai.2014.59.247 RESEARCH ARTICLE Abiotic Factors and Yushania Influences on Abies Forest Composition in Taiwan Cheng-Tao Lin(1), Tzu-Ying Chen(2), Chang-Fu Hsieh(3) and Chyi-Rong Chiou(1*) 1. School of Forestry and Resource Conservation, National Taiwan University, No. 1, Sect. 4, Roosevelt Rd., Taipei, 10617, Taiwan. 2. Department of Forestry and Natural Resources, National Ilan University, Sect. 1, Shen-Lung Rd., Ilan, 26047, Taiwan. 3. Institute of Ecology and Evolutionary Biology, National Taiwan University, No. 1, Sect. 4, Roosevelt Rd., Taipei, 10617, Taiwan. * Corresponding author. Tel.: +886-2-3366-4640; Fax: +886-2-2365-4520; Email: [email protected] (Manuscript received 20 March 2014; accepted 26 May 2014) ABSTRACT: Abies kawakamii forests are generally distributed above 3,000 m in Taiwanese high mountains. The community data used in our analysis were derived from the database of the National Vegetation Diversity Inventory and Mapping Project of Taiwan (NVDIMP), and environmental data were obtained from the WorldClim and NVDIMP databases. We used non-metric multidimensional scaling (NMDS) to identify vegetation composition of Abies communities and the structural equation models (SEMs) were used to examine the complex relationships between environmental factors and vegetation composition. The results of ordination showed the most important factors determining species composition of Abies forests involved habitat rockiness, heat load index, warmth index and summer and winter. SEM results approved the warmth index and winter precipitation were the main drivers determining the latent variable—climate, which significantly affect the overstory composition of Abies communities. -

Anthropogenic Fire, Vegetation Structure and Ethnobotanical Uses in an Alpine Shrubland of Nepal’S Himalaya
CSIRO PUBLISHING International Journal of Wildland Fire 2020, 29, 201–214 https://doi.org/10.1071/WF19098 Anthropogenic fire, vegetation structure and ethnobotanical uses in an alpine shrubland of Nepal’s Himalaya Asha PaudelA,B,F, Scott H. MarkwithB, Katie KoncharC, Mani Shrestha D,E and Suresh K. GhimireA,F ACentral Department of Botany, Tribhuvan University, Kathmandu, 44618, Nepal. BDepartment of Geosciences, Florida Atlantic University, 777 Glades Road, Boca Raton, FL, 33431, USA. C1334 Jackson Street, Tallahassee, FL, 32301, USA. DSchool of Media and Communication, RMIT University, Melbourne, Vic. 3001, Australia. EFaculty of Information Technology, Monash University, Melbourne, Vic. 3800, Australia. FCorresponding authors. Email: [email protected], [email protected] Abstract. Alpine vegetation of the Himalaya is used as food, medicine or fodder, and is commonly managed with fire by agropastoralists. Prescribed fire can have positive effects on rangeland biodiversity, but studies evaluating its effects in alpine shrublands are scarce. Our objective was to examine the effects of anthropogenic fire on biophysical characteristics, species richness, abundance and composition in an alpine shrubland with socioeconomic value to local peoples in Langtang National Park in central Nepal. We surveyed biophysical variables, vascular plant species richness and composition along three transects at ascending elevations, and conducted interviews with local people and park officials on the use of fire in the region. We found 69 species of vascular plants in 89 plots; species richness was greater in burned plots and with increasing elevation, with 13 species unique to burned plots. We identified 14 indicator species in both burned and unburned plots; eight of them were Himalayan endemics. -

Building a Reference Transcriptome for Juniperus Squamata (Cupressaceae) Based on Single-Molecule Real-Time Sequencing
Building a reference Transcriptome for Juniperus squamata (Cupressaceae) based on Single-molecule real-time sequencing Yufei Wang Sichuan University School of Life Sciences Siyu Xie Sichuan University School of Life Sciences Jialiang Li College of Life Sciences, Sichuan University Jieshi Tang College of Life Science, Sichuan University Tsam Ju College of Life Sciences, Sichuan University Kangshan Mao ( [email protected] ) Sichuan University https://orcid.org/0000-0002-0071-1844 Data note Keywords: Juniperus squamata, single-molecule real-time sequencing, simple sequence repeats Posted Date: May 25th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-548946/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/5 Abstract Objectives Cupressaceae is the second largest family of coniferous trees (Coniferopsida) with important economic and ecological values. However, like other conifers, the members of Cupressaceae have extremely large genome (>8 gigabytes), which limited the researches of these taxa. A high-quality transcriptome is an important resource for gene discovery and annotation for non- model organisms. Data description Juniperus squamata, a tetraploid species which is widely distributed in Asian mountains, represents the largest genus, Juniperus, in Cupressaceae. Single-molecule real-time sequencing was used to obtain full-length transcriptome of Juniperus squamata. The full-length transcriptome was corrected with Illumina RNA-seq data from the same individual. A total of 47, 860 non-redundant full-length transcripts, N50 of which was 2, 839, were obtained. Simple sequence repeats for Juniperus squamata were also identied. This data presents the rst comprehensive transcriptome characterization of Cupressaceae species, and provides an important reference for researches on the genomic evolutionary history of Cupressaceae plants and even conifers in the future. -

Variation in Leaf Biomass and Fruit Output of Juniperus Indica Along an Elevation Gradient in North-Central Nepal A
Variation in leaf biomass and fruit output of Juniperus indica along an elevation gradient in north-central Nepal A. Chapagain1, 3, R. P. Chaudhary2 and S. K. Ghimire3 Biomass and reproductive output are important functional traits that influence aspects of plant performance. Measurements of these attributes by harvesting plant parts are often destructive and impractical. Therefore, non-destructive methods, based on allometric relationships, have been recommended for measuring plant biomass and reproductive output, particularly in the ecosystems where plant harvesting is not very practical or feasible. Here, we assessed the variation in the traits related to vegetative and reproductive performance (including plant height, trunk diameter, canopy area, leaf biomass and number of fruits set) among populations of Juniperus indica distributed along an elevation gradient in Manang district of the north-central Nepal, and finally determined the allometric relationships addressing the leaf biomass and the fruit output. The distribution range of J. indica was divided into lower- (3,350‒3,580m), mid- (3,650‒3,880m) and higher- (3,950‒4,250m) elevation classes where we made 54 sample plots of 10m × 10m size. In each plot, we recorded the number of individuals of J. indica classifying into seedling, juvenile and mature classes, and measured their vegetative traits and fruit output. Trunk diameter, leaf dry-weight and fruits set parameters spatially varied within the same elevation class. The individuals at the lower-elevation were larger in vegetative size with larger- trunk, height and canopy area, and produced higher leaf biomass and greater number of fruits as compared to those produced by the individuals situated at the mid- and higher-elevations. -

Juniper (Juniperus Davurica ‘Parsonii’, J
Juniper (Juniperus davurica ‘Parsonii’, J. conferta 'Blue Pacific', J. squamata 'Blue Star') Tolerance to Glyphosate. Mark Andrew Czarnota Department of Horticulture - Griffin The University of Georgia NATURE OF WORK: To evaluate the tolerance of 3 juniper species to varying rates of glyphosate. For many reasons preemergent herbicides can fail to control weeds. Once weed seeds germinate, nurseryman and landscapers are often left with hand weeding as their only control option. Including junipers, many plants are known to be very tolerant to glyphosate. Moreover, glyphosate can control a wide number of herbaceous plants at 0.5 lbs. ai./A and lower. The goal of the test is to determine the maximum amount of glyphosate that can be applied without damaging select juniper species. MATERIAL AND METHODS: On July 27, 2003 at the Center for Applied Nursery Research, 18, 1 gallon pots of each of the following junipers were assambled: Shore juniper (Juniperus conferta 'Blue pacific'), Dahurian juniper (J. davurica 'Parsoni'), Singleseed juniper (J. squamata Blue Star'). Nine, one gallon pots, of each species were placed in a 6 ft. x 6 ft. area. Herbicide treatments were applied to the area containing the 27 one gallon pots. Pots were then carefully moved to an evaluation area were they were arranged in a randomized complete block (RCB) design. Each treatment contained 3 replications, and each replication contained 3 subsamples. The process was continued for each herbicide treatment. Sprays were applied with a CO2 backpack sprayer calibrated to deliver 20 gallons per acer (GPA). Watering occurred on an as needed basis, and this represented approximately ½ to 1 inch of water per day. -

Vascular Plants of Virginia Lake Reserve, St John's Hill, Wanganui
Vascular Plants of Virginia Lake Reserve, St John’s Hill, Wanganui Wanganui Plant List No. 99 (incorporating Wanganui Plant List No. 78: wetland plants of Virginia Lake) Compiled by Colin Ogle Department of Conservation, Wanganui With assistance of members of Wanganui Botanical Group, and extra identifications by C Ecroyd (FRI Rotorua); E Cameron and R Gardner (Auckland Museum); P Simpson (Lincoln) Last updated 19 Dec 2000 For exotic species, * denotes planted specimens and ** denotes species that are self- establishing, including weeds and species spreading from planted specimens. For NZ indigenous species, “a” denotes species presumed to be natural to the reserve; “b” denotes planted specimens, and “c” denotes planted specimens that are now self- establishing. CHR,NZFRI, AK: specimens from Virginia Lake in herbaria at Landcare Research, Lincoln (CHR); Rotorua (NZFRI); Auckland Museum (AK) Trees and shrubs 1. Gymnosperms * Abies nordmanniana Caucasian fir b Agathis australis kauri * Araucaria araucana monkey puzzle * Araucaria heterophylla Norfolk pine * Callitris gracilis ssp. gracilis (NZFRI * Callitris rhomboidea (NZFRI Port Jackson pine, cypress pine * Cedrus atlantica ‘glauca’ Atlantic cedar * Cedrus sp. (?C. deodara/libaii) cedar * Chamaecyparis funebris (NZFRI 22551) * Chamaecyparis lawsoniana (NZFRI 22550) Lawson’s cypress * Cryptomeria japonica Japanese cedar * Cunninghamia lanceolata Chinese fir * Cupressus lusitanica (NZFRI) * Cupressus macrocarpa macrocarpa * Cupressus sempervirens (NZFRI 22546, 23208) Italian cypress b Dacrycarpus dacrydioides kahikatea b Dacrydium cupressinum rimu * Decussocarpus falcatus (= Afrocarpus falcata) (has bark like matai) (NZFRI 8391, 22437, 23301) * Gingko biloba gingko, maidenhair tree * Juniperus chinensis (NZFRI Chinese juniper * Juniperus communis ‘ ‘ juniper * Juniperus recurva (NZFRI * Juniperus squamata ‘Meyeri’ flaky juniper * Metasequoia glyptostroboides (NZFRI 22467) dawn redwood * Picea abies [ID conf. -

National Register of Medicinal Plants
Digitized by Google Digitized by Google IUCI Nepal National Register of Medicinal Plants IUCl-The World Conservation union May 2000 ... .....,...... , ... 111 IUCN ....,, ., fllrlll •• ... c-.ltloll n.w.wc:-....u.i. IIHI l111I11111I1111II1111II111111111111111 9AZG-Y9Q-23PK Published by: IUCN Nepal Copyright: 2000. IUCN Nepal The role of Swiss Agency for Development and Cooperation in supporting the IUCN Nepal is gratefully acknowledged. The material in this publication may be reproduced in whole or in part and in any form for education or non-profit uses, without special permission from the copyright holder, provided acknowledgment of the source is made. IUCN Nepal would appreciate receiving a copy of any publication which uses this publication as a source. No use of this publication may be made for resale or other commercial purposes without prior written permission of IUCN Nepal. Citation: IUCN Nepal. 2000. National Register ofMedicinal Plants. Kathmandu: IUCN Nepal. ix+ 163 pp. ISBN: 92-9144-048-5 Layout and Design: Upendra Shrestha & Kanhaiya L. Shrestha Cover design: Upendra Shrestha Cover Pictures: Pages from the manuscript of Chandra Nighantu drawn towards the end of 19th century (Courtesy: Singh Durbar Vaidhyakhana Development Committee) Left-hand side: Rajbriksha (Cassia fistula) occuring in the Tarai and other tropical regions of Nepal lying below 1,000 m altitude. Right-hand side: jatamansi (Nardostachys grandif/ora) occuring at 3,000m to 4,000m in the alpine and subalpine zone of Nepal Himalaya. Available from: IUCN Nepal P.O. Box 3923 Kathmandu, Nepal The views expressed in this document are those of the authors and do not necessarily reflect the official views of IUCN Nepal.