Cone Morphology in Juniperus in the Light of Cone Evolution in Cupressaceae S.L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Analyses of Juniperus Species in Turkey and Their Relations with Other Juniperus Based on Cpdna Supervisor: Prof
MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY AYSUN DEMET GÜVENDİREN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY APRIL 2015 Approval of the thesis MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA submitted by AYSUN DEMET GÜVENDİREN in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Department of Biological Sciences, Middle East Technical University by, Prof. Dr. Gülbin Dural Ünver Dean, Graduate School of Natural and Applied Sciences Prof. Dr. Orhan Adalı Head of the Department, Biological Sciences Prof. Dr. Zeki Kaya Supervisor, Dept. of Biological Sciences METU Examining Committee Members Prof. Dr. Musa Doğan Dept. Biological Sciences, METU Prof. Dr. Zeki Kaya Dept. Biological Sciences, METU Prof.Dr. Hayri Duman Biology Dept., Gazi University Prof. Dr. İrfan Kandemir Biology Dept., Ankara University Assoc. Prof. Dr. Sertaç Önde Dept. Biological Sciences, METU Date: iii I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name : Aysun Demet GÜVENDİREN Signature : iv ABSTRACT MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA Güvendiren, Aysun Demet Ph.D., Department of Biological Sciences Supervisor: Prof. -

Morphology and Morphogenesis of the Seed Cones of the Cupressaceae - Part II Cupressoideae
1 2 Bull. CCP 4 (2): 51-78. (10.2015) A. Jagel & V.M. Dörken Morphology and morphogenesis of the seed cones of the Cupressaceae - part II Cupressoideae Summary The cone morphology of the Cupressoideae genera Calocedrus, Thuja, Thujopsis, Chamaecyparis, Fokienia, Platycladus, Microbiota, Tetraclinis, Cupressus and Juniperus are presented in young stages, at pollination time as well as at maturity. Typical cone diagrams were drawn for each genus. In contrast to the taxodiaceous Cupressaceae, in Cupressoideae outgrowths of the seed-scale do not exist; the seed scale is completely reduced to the ovules, inserted in the axil of the cone scale. The cone scale represents the bract scale and is not a bract- /seed scale complex as is often postulated. Especially within the strongly derived groups of the Cupressoideae an increased number of ovules and the appearance of more than one row of ovules occurs. The ovules in a row develop centripetally. Each row represents one of ascending accessory shoots. Within a cone the ovules develop from proximal to distal. Within the Cupressoideae a distinct tendency can be observed shifting the fertile zone in distal parts of the cone by reducing sterile elements. In some of the most derived taxa the ovules are no longer (only) inserted axillary, but (additionally) terminal at the end of the cone axis or they alternate to the terminal cone scales (Microbiota, Tetraclinis, Juniperus). Such non-axillary ovules could be regarded as derived from axillary ones (Microbiota) or they develop directly from the apical meristem and represent elements of a terminal short-shoot (Tetraclinis, Juniperus). -

Genetic Structure of Tetraclinis Articulata, an Endangered Conifer of the Western Mediterranean Basin
Silva Fennica vol. 47 no. 5 article id 1073 Category: research article SILVA FENNICA www.silvafennica.fi ISSN-L 0037-5330 | ISSN 2242-4075 (Online) The Finnish Society of Forest Science The Finnish Forest Research Institute Pedro Sánchez-Gómez1, Juan F. Jiménez1, Juan B. Vera1, Francisco J. Sánchez-Saorín2, Juan F. Martínez2 and Joseph Buhagiar3 Genetic structure of Tetraclinis articulata, an endangered conifer of the western Mediterranean basin Sánchez-Gómez P., Jiménez J. F., Vera J. B., Sánchez-Saorín F. J., Martínez J. F., Buhagiar J. (2013). Genetic structure of Tetraclinis articulata, an endangered conifer of the western Mediter- ranean basin. Silva Fennica vol. 45 no. 5 article id 1073. 14 p. Highlights • The employment of ISSR molecular markers has shown moderate genetic diversity and high genetic differentiation in Tetraclinis articulata. • Genetic structure of populations seems to be influenced by the anthropogenic use of this species since historical times, or alternatively, by the complex palaeogeographic history of the Mediterranean basin. • Results could be used to propose management policies for conservation of populations. Abstract Tetraclinis articulata (Vahl) Masters is a tree distributed throughout the western Mediterranean basin. It is included in the IUCN (International Union for Conservation of Nature) red list, and protected by law in several of the countries where it grows. In this study we examined the genetic diversity and genetic structure of 14 populations of T. articulata in its whole geographic range using ISSR (inter simple sequence repeat) markers. T. articulata showed moderate genetic diversity at intrapopulation level and high genetic differentiation. The distribution of genetic diversity among populations did not exhibit a linear pattern related to geographic distances, since all analyses (principal coordinate analysis, Unweighted pair group method with arithmetic mean dendrogram and Bayesian structure analysis) revealed that spanish population grouped with Malta and Tunisia populations. -
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -
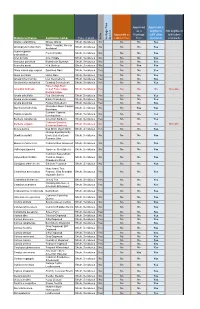
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -
Dwarf Conifers Bitner, Richard L., Conifers for Gardens: an Illustrated Encyclopedia
TO LEARN MORE gardening with dwarf conifers Bitner, Richard L., Conifers for Gardens: An Illustrated Encyclopedia. Portland, OR: Timber Press, 2007. Dwarf conifers are ideal plants for Chicagoland’s smaller yards Cutler, Sandra McLean, Dwarf & and gardens. Th ey’re low- Unusual Conifers Coming of Age. maintenance and resistant to Washington, D.C.: United States most insects and diseases; many National Arboretum, 1997. have year-round color; and Krüssmann, Gert, Manual of there is an extraordinary range of sizes and shapes available. Cultivated Conifers. Portland, OR: Timber Press, 1986. One of the best of its kind in the country, the Dwarf Van Gelderen, D.M. and Van Hoey Smith, J.R.P., Conifers: Th e Conifer Garden showcases more than 150 diff erent kinds Illustrated Encyclopedia. Portland, OR: Timber Press, 1996. of the smaller members of the conifer family. Renovated in Growth rates dwarf conifer 2008 (it was fi rst dedicated in 1988), the garden includes Dwarf conifers grow slowly. Th e American Conifer Th e American Conifer Society website off ers in-depth informa- a new staircase entrance, views to the Japanese Garden and Society off ers these size guidelines for all conifers: selections tion about conifers at www.conifersociety.org. Great Basin, and a widened, accessible-to-all path. Dwarf conifers for blue color Lenhardt Library Category Approximate Approximate Conifers are plants that bear cones. Mostly native to the What humans see as blue color is actually a protective, For these and other titles, visit the Lenhardt Library of the growth per year size at 10 years earth’s northern hemisphere, conifers have skinny needle- waxy outer coating on new-growth needles. -

Supporting Information
Supporting Information Mao et al. 10.1073/pnas.1114319109 SI Text BEAST Analyses. In addition to a BEAST analysis that used uniform Selection of Fossil Taxa and Their Phylogenetic Positions. The in- prior distributions for all calibrations (run 1; 144-taxon dataset, tegration of fossil calibrations is the most critical step in molecular calibrations as in Table S4), we performed eight additional dating (1, 2). We only used the fossil taxa with ovulate cones that analyses to explore factors affecting estimates of divergence could be assigned unambiguously to the extant groups (Table S4). time (Fig. S3). The exact phylogenetic position of fossils used to calibrate the First, to test the effect of calibration point P, which is close to molecular clocks was determined using the total-evidence analy- the root node and is the only functional hard maximum constraint ses (following refs. 3−5). Cordaixylon iowensis was not included in in BEAST runs using uniform priors, we carried out three runs the analyses because its assignment to the crown Acrogymno- with calibrations A through O (Table S4), and calibration P set to spermae already is supported by previous cladistic analyses (also [306.2, 351.7] (run 2), [306.2, 336.5] (run 3), and [306.2, 321.4] using the total-evidence approach) (6). Two data matrices were (run 4). The age estimates obtained in runs 2, 3, and 4 largely compiled. Matrix A comprised Ginkgo biloba, 12 living repre- overlapped with those from run 1 (Fig. S3). Second, we carried out two runs with different subsets of sentatives from each conifer family, and three fossils taxa related fi to Pinaceae and Araucariaceae (16 taxa in total; Fig. -

Abiotic Factors and Yushania Influences on Abies Forest Composition in Taiwan
Taiwania, 59(3): 247‒261, 2014 DOI: 10.6165/tai.2014.59.247 RESEARCH ARTICLE Abiotic Factors and Yushania Influences on Abies Forest Composition in Taiwan Cheng-Tao Lin(1), Tzu-Ying Chen(2), Chang-Fu Hsieh(3) and Chyi-Rong Chiou(1*) 1. School of Forestry and Resource Conservation, National Taiwan University, No. 1, Sect. 4, Roosevelt Rd., Taipei, 10617, Taiwan. 2. Department of Forestry and Natural Resources, National Ilan University, Sect. 1, Shen-Lung Rd., Ilan, 26047, Taiwan. 3. Institute of Ecology and Evolutionary Biology, National Taiwan University, No. 1, Sect. 4, Roosevelt Rd., Taipei, 10617, Taiwan. * Corresponding author. Tel.: +886-2-3366-4640; Fax: +886-2-2365-4520; Email: [email protected] (Manuscript received 20 March 2014; accepted 26 May 2014) ABSTRACT: Abies kawakamii forests are generally distributed above 3,000 m in Taiwanese high mountains. The community data used in our analysis were derived from the database of the National Vegetation Diversity Inventory and Mapping Project of Taiwan (NVDIMP), and environmental data were obtained from the WorldClim and NVDIMP databases. We used non-metric multidimensional scaling (NMDS) to identify vegetation composition of Abies communities and the structural equation models (SEMs) were used to examine the complex relationships between environmental factors and vegetation composition. The results of ordination showed the most important factors determining species composition of Abies forests involved habitat rockiness, heat load index, warmth index and summer and winter. SEM results approved the warmth index and winter precipitation were the main drivers determining the latent variable—climate, which significantly affect the overstory composition of Abies communities. -

Building a Reference Transcriptome for Juniperus Squamata (Cupressaceae) Based on Single-Molecule Real-Time Sequencing
Building a reference Transcriptome for Juniperus squamata (Cupressaceae) based on Single-molecule real-time sequencing Yufei Wang Sichuan University School of Life Sciences Siyu Xie Sichuan University School of Life Sciences Jialiang Li College of Life Sciences, Sichuan University Jieshi Tang College of Life Science, Sichuan University Tsam Ju College of Life Sciences, Sichuan University Kangshan Mao ( [email protected] ) Sichuan University https://orcid.org/0000-0002-0071-1844 Data note Keywords: Juniperus squamata, single-molecule real-time sequencing, simple sequence repeats Posted Date: May 25th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-548946/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/5 Abstract Objectives Cupressaceae is the second largest family of coniferous trees (Coniferopsida) with important economic and ecological values. However, like other conifers, the members of Cupressaceae have extremely large genome (>8 gigabytes), which limited the researches of these taxa. A high-quality transcriptome is an important resource for gene discovery and annotation for non- model organisms. Data description Juniperus squamata, a tetraploid species which is widely distributed in Asian mountains, represents the largest genus, Juniperus, in Cupressaceae. Single-molecule real-time sequencing was used to obtain full-length transcriptome of Juniperus squamata. The full-length transcriptome was corrected with Illumina RNA-seq data from the same individual. A total of 47, 860 non-redundant full-length transcripts, N50 of which was 2, 839, were obtained. Simple sequence repeats for Juniperus squamata were also identied. This data presents the rst comprehensive transcriptome characterization of Cupressaceae species, and provides an important reference for researches on the genomic evolutionary history of Cupressaceae plants and even conifers in the future. -

GC-MS Analysis for Polyaromatic Hydrocarbons (PAH) in Moroccan Medicinal Tars
GC-MS analysis for Polyaromatic Hydrocarbons (PAH) in Moroccan medicinal tars An ethnobotanical study and chemical investigation of the use and safety of medicinal tars in Marrakesh and the High Atlas Mountains, Morocco Marcus Lindborg Arbetsgruppen för Tropisk Ekologi Minor Field Study 136 Committee of Tropical Ecology ISSN 1653-5634 Uppsala University, Sweden January 2009 Uppsala GC-MS analysis for Polyaromatic Hydrocarbons (PAH) in Moroccan medicinal tars An ethnobotanical study and chemical investigation of the use and safety of medicinal tars in Marrakesh and the High Atlas Mountains, Morocco Marcus Lindborg Degree project in biology, Master of science (1 year), 2008 Examensarbete i biologi 30 hp till magisterexamen, 2008 Biology Education Centre and Department of Systematic Botany, Uppsala University Supervisors: Lars Björk and Hugo de Boer Abstract Medicinal tar is a reddish-brown liquid with a smoky odour, which is traditionally produced through pyrolysis of trunks or roots of different coniferous trees, e.g. Juniperus oxycedrus, Juniperus phoenicea, Juniperus thurifera, Tetraclinis articulata and Cedrus atlantica. Trade and use of medicinal tars in Europe and North America is restricted due to potential carcinogenicity. The only presently allowed uses are as a fragrance substance in cosmetics. This study carried out semi-structured interviews with producers, herbalists and traditional midwives/healers (ferraga) in the High Atlas mountains of Morocco, to assess saliency of use, what species are used and how medicinal tar is used in the Marrakesh region. We found that Juniperus phoenicea and Juniperus oxycedrus are used most frequently for tar production. Frequent trade was reported by retailers, and the traditional herbal intermediaries (both herbalists and ferraga) report that tar is used most commonly for hair care, skin diseases and fumigation. -

Makhtar Cypress & Atlas Cypress
Makhtar Cypress & Atlas Cypress Cupressus sempervirens var. numidica Trabut. Bou Abdallah – Tunisia Cupressus atlantica Gaussen. Sidi Youssef ou Brahim – Morocco he forests of Mediterranean cypress in northern Africa can be The Atlas Cypress are a relict species of the high western found in two locations: northeast Makhtar on the mountains Atlas Mountains of Morocco, which according to the FAO, Tforming the backbone of Tunisia, and the Cyrenaica’s Green is now genetically impoverished. Its populations diminished Mountain in eastern Libya. The only population in Tunisia can sharply throughout the 20th century as a result of forestry over- be found on the Kessra plateau, made up of a small group of exploitation, intensive grazing and the local use of its wood. extremely old trees, heavily mutilated and shaped irregularly, The Aghbar forest, with an area of 2,800 hectares, is the most which survive in rocky, eroded soil. The Makhtar cypress are extensive and best-preserved forest. The rest forms small islands regarded as an endemic Tunisian variant of the Mediterranean of vegetation in a very advanced state of degradation and limited cypress. regeneration. Red List Category of Threatened Species, IUCN: Endangered (EN). n n 96 97 n Junipers of Kedrodasos Juniperus oxycedrus L. var. macrocarpa (Sibth. & Sm.) Silba Juniperus phoenicea L. Elafonisi – Crete – Greece he azonal formations of junipers on the coastal dunes of the thermo-Mediterranean belt appear to be associated Twith edaphic determinism. These coastal juniper woods, made up of different taxa of the Phoenicea group, can form very dense forestry masses in which individual trees can grow to heights of more than 20 metres. -

Synanthropic Flora of Petra (Jordan)
Synanthropic flora of the archaeological sites of Petra (Jordan) Prof. Dr. Dietmar Brandes http://www.ruderal-vegetation.de/verschiedene_regionen/index.htm Petra – Ancient capital of the Nabateans • The most famous place of Jordan is Petra, the former capital of the Nabateans. The hidden location, the safe water supply and the position at the crossing of the Incense Route and the overland route from Egypt to India gave the Nabateans a hold over the trade. • Some 800 monuments are still to be found in the area. In 1985 Petra was designated a World Heritage Site; in 2007 it was named one of the New Seven Wonders of the World. • Petra (30 19‘ 44‘‘N 35 26‘ 25‘‘O) is situated in an altitude between 800 and 1.350 m. • From the biogeographical point of view it is near the crossing of the Mediterranean region, the Irano- Turanion region, the Saharo-Arabian and the Sudanian region. Khazne al-Firaun „Treasury“ • The access to the ancient city of Petra leads through the very impressive Siq, a dim gorge in the Umm Ishrin sandstone. • The Siq has a length of 1.200 m, the walls are 90-180 m high. Synanthropic flora of the Siq Adiantum capillus-veneris, Anchusa strigosa, Ajuga chia, Arum elongatum, Ballota cf. Undulata, Bryonia, Capparis spinosa, Carduus cf. argentatus, Daphne linearifolia, Diplotaxis harra, Echinops, Emex spinosa, Ephedra, Ferula communis, Ficus carica, Galium aparine, Gomphocarpus sinaicus, Hyoscyamus aureus, Juniperus phoenicea, Lamarckia aurea, Leopoldia comosa, Malva parviflora, Malva sylvestris, Marrubium vulgare, Matthiola longipetala, Nerium oleander, Parietaria alsinifolia, Parietaria judaica, Phlomis, Phragmites australis, Podonosma orientalis, Salvia lanigera, Sanguisorba cf.