Delivering Our Pipeline Through Scientific Leadership
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Research Council
OFFICIAL MEDICAL RESEARCH COUNCIL 03 Should not be viewed by CEO due to Author: Sam Bartholomew Conflict of interest Should not be viewed by deputy CEO due to Conflict of interest Trade Union Side access: In part Minutes of the Council meeting held at the Chelsea Harbour Hotel on 7 March 2018 Present: Council Head Office staff Mr Donald Brydon (Chair) Ms Sam Bartholomew Sir John Savill (CEO) Professor David Lomas Professor Jonathan Bisson Dr Rob Buckle Dr John Brown Mr Hugh Dunlop Professor Doreen Cantrell Dr Declan Mulkeen Professor Chris Day Dr Tony Peatfield Professor Dame Janet Finch Mrs Helen Page Professor John Iredale Dr Rachel Quinn Mr Richard Murley Dr Frances Rawle Baroness Onora O’Neill Ms Pauline Mullin (item 9a&b only) Dr Mene Pangalos Professor Irene Tracey Guests – item 8 Dr Pauline Williams Nick Lemoine, Chair MRF Trust Angela Hind, CEO MRF Observers Ms Jenny Dibden (BEIS) Professor Fiona Watt, MRC Executive Chair Designate 1. Announcements Mr Brydon welcomed members to the last meeting of the MRC Council as currently constituted, prior to the transition to UK Research and Innovation. He introduced Professor Fiona Watt, MRC Executive Chair Designate, and Professor Jonathan Bisson who was attending his first meeting since being appointed as a Council member in the summer. He offered congratulations to those MRC-associated people whose achievements had been recognised in the New Year’s Honours: • Sir Keith Peters Sir Keith, lately Regius Professor of Physic at the University of Cambridge, was appointed Knight Grand Cross of the Order of the British Empire (GBE) for services to 1 OFFICIAL the advancement of medical science. -

Alderley Park the Future As a World-Class Life Science Facility Planning Application Summary Document
DECEMBER 2015 ALDERLEY PARK THE FUTURE AS A WORLD-CLASS LIFE SCIENCE FACILITY PLANNING APPLICATION SUMMARY DOCUMENT Alderley Park is a world-class Life Science research and development (R&D) facility. The site currently houses around 190,000 sq m of premier scientific laboratory space, offices, restaurants, cafes, conferencing space and energy plant, set in 400 acres of parkland. As a centre for scientific research, it is one of the few facilities in the country with a comprehensive offering to support drug discovery and development. It was AstraZeneca’s global lead centre for cancer research, employing at its peak up to 10,000 people in highly skilled jobs. These facilities at Alderley Park are high-quality A planning application has been submitted to and high-tech, and require significant ongoing Cheshire East Council for future development investment to retain them. £550 million has been at Alderley Park. The planning application invested in Alderley Park since 1997 and further, has been allocated the reference number major investment is required to repurpose the site 15/5401M. from single to multi-occupier use, maintain the facilities and cover ongoing overheads. This summary document sets out the key elements of the proposals. ILLUSTRATIVE MASTERPLAN SIGNIFICANT INVESTMENT PROPOSED OVER THE NEXT 10 YEARS In March 2013, AstraZeneca announced The site was purchased by Bruntwood/MSP, its decision to relocate its R&D facilities to part of Alderley Park Ltd who now wish to Cambridge, leading to uncertainty over the secure the future of the site, retaining talent and future of Alderley Park and its role in the local jobs, by taking forward the Taskforce’s vision. -

Introducing the IMED Biotech Unit What Science Can Do Introduction What Science Can Do
Introducing the IMED Biotech Unit What science can do Introduction What science can do At AstraZeneca, our purpose is to push the Our IMED Biotech Unit applies its research and Our approach to R&D development capabilities and technologies to The IMED Biotech Unit plays a critical boundaries of science to deliver life-changing accelerate the progress of our pipeline. Through role in driving AstraZeneca’s success. Working together with MedImmune, medicines. We achieve this by placing science great collaboration across our three science units, our global biologics arm and Global we are confident that we can deliver the next wave Medicines Development (GMD), our at the centre of everything we do. late-stage development organisation, of innovative medicines to transform the lives of we are ensuring we deliver an innovative patients around the world. and sustainable pipeline. Pancreatic beta cells at different Eosinophil prior to Minute pieces of circulating tumour DNA stages of regeneration apoptosis (ctDNA) in the bloodstream IMED Biotech Unit MedImmune Global Medicines Development Focuses on driving scientific advances Focuses on biologics research and Focuses on late-stage development in small molecules, oligonucleotides and development in therapeutic proteins, of our innovative pipeline, transforming other emerging platforms to push the monoclonal antibodies and other next- exciting science into valued new boundaries of medical science. generation molecules to attack a range medicines and ensuring patients of diseases. around the world can access them. It’s science that compels us to push the boundaries of what is possible. We trust in the potential of ideas and pursue them, alone and with others, until we have transformed the treatment of disease. -

Novel Neutralizing Hedgehog Antibody MEDI-5304 Exhibits Antitumor Activity by Inhibiting
Author Manuscript Published OnlineFirst on December 16, 2013; DOI: 10.1158/1535-7163.MCT-13-0420 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Fully Human Anti-Hedgehog Antibodies Novel neutralizing hedgehog antibody MEDI-5304 exhibits antitumor activity by inhibiting paracrine hedgehog signaling. Authors: Neil R. Michaud1*, Youzhen Wang1, Kristen McEachern1, Jerold J. Jordan1, Anne Marie Mazzola1, Axel Hernandez1, Sanjoo Jalla2, Jon W. Chesebrough2, Mark J. Hynes3, Matthew Belmonte 1, Lidong Wang3, Jaspal S. Kang4#, Jelena Jovanović5, Naomi Laing1, David W. Jenkins1, Elaine Hurt2, Meina Liang6, Christopher Frantz7, Robert E. Hollingsworth2, Diane M. Simeone3, David C. Blakey8, and Vahe Bedian1* Authors’ affiliation: 1Oncology iMED, AstraZeneca-R&D Boston, Waltham, MA 2Oncology Research, MedImmune LLC, Gaithersburg, MD 3Translational Oncology Program, University of Michigan, Ann Arbor, MI; 4Amgen British Columbia, Burnaby, BC, Canada 5Lead Generation- Research, Medimmune LLC, Granta Park, Cambridge, UK 6Clinical Pharmacology and DMPK, MedImmune LLC, Hayward, CA 7Biologics Safety Assessment, MedImmune LLC, Mountain View, CA 8Oncology iMed, AstraZeneca, Alderley Park, Macclesfield. UK #Current address: Paul Kang, CSO, Innovative Targeting Solutions Inc., Burnaby BC, Canada *corresponding authors: Neil R. Michaud, Ph.D. Oncology iMED AstraZeneca-R&D Boston Waltham, MA 02451 e-mail: [email protected] Vahe Bedian Ph.D. Oncology iMED AstraZeneca-R&D Boston Waltham, MA 02451 phone:781-839-4613 e-mail: [email protected] Running title: Fully Human Anti-Hedgehog Antibodies Key Words: Hedgehog, Antibody, Xenograft, XenoMouse, cancer stem cells Grant Support: This work was funded by AstraZeneca PLC and MedImmune LLC. Disclosure of Potential Conflicts of Interest: There are no potential conflicts of interest. -

Scientific Insights in a Pandemic COVID-19 National Core Studies Symposium 24 June 2021
Scientific Insights in a Pandemic COVID-19 National Core Studies Symposium 24 June 2021 Agenda Session title and speaker 09:00 Opening keynote: A year of accelerated science and impacts Jeremy Farrar, Wellcome Trust 09.10 UK COVID Science, the public and the media • Fiona Fox, Science Media Centre • Chris Monk, public representative 09.20 Scientific impact on the NHS and patients Mark Toshner, Cambridge University Hospitals NHS Foundation Trust 09.30 Plenary keynote: What we have learned from COVID? Patrick Vallance, Chief Scientific Advisor UK Government 09.50 National Core Studies – impact and future potential • Transmission and environment – Andrew Curran • Longitudinal health and wellbeing – Nish Chaturvedi and Jonathan Sterne • Immunity – Paul Moss • Epidemiology and Surveillance – Ian Diamond • Vaccines Trials Infrastructure – Divya Chadha Manek • Therapeutics Trials Infrastructure – Patrick Chinnery • Data and Connectivity – Andrew Morris Chaired by Anne Johnson Q&A 11.10 Break 11.20 Lightning talks - Scientific Impact of UK COVID science 11.20 Group 1 Group 2 • Anne-Marie Docherty, University of • Dinesh Aggarwal, University of Edinburgh Cambridge • Marion Mafham, Nuffield Department of • Helen Parry, NIHR Population Health, University of Oxford • Joshua Elliott, Imperial College London • Muzz Hanifa, Newcastle University • William Hulme, Oxford DataLab • Cameron Razieh, University of Leicester Q&A Q&A 12.10 Preparing for the next pandemic • Mene Pangalos, AstraZeneca • Gabriel Leung, University of Hong Kong • Lynda Stuart, Bill & Melinda Gates Foundation • Further speakers TBC 12:35 International Guest lecture - Opportunities for global collaborative open science Keynote speaker TBC Fireside chat with Ottoline Leyser, Chief Executive, UK Research and Innovation 12:55 Closing remarks Patrick Vallance, Government Chief Scientific Adviser 13:00 End . -

Council for Scientific and Industrial Research
ANNUAL 2018/19 PO Box 395, Pretoria, 0001, South Africa Published by: CSIR Communication Enquiries: Tel +27 12 841 2911 • Email: [email protected] ISBN-13 978-0-7988-5643-0 “The objects of the CSIR are, through directed and particularly multi-disciplinary research and technological innovation, to foster, in the national interest and in fields which in its opinion should receive preference, industrial and scientific development, either by itself or in co-operation with principals from the private or public sectors, and thereby to contribute to the improvement of the quality of life of the people of the Republic, and to perform any other functions that may be assigned to the CSIR by or under this Act.” (Scientific Research Council Act 46 of 1988, as amended by Act 27 of 2014) CONTENTS The CSIR at a glance ......................................................2 From our leadership .......................................................6 Organisational highlights .............................................. 24 Financial sustainability and governance .......................... 66 Executive report ........................................................... 87 Consolidated financial statements ................................ 101 Knowledge dissemination ............................................ 148 Abbreviations ............................................................ 175 1 INTRODUCTION THE CSIR AT A GLANCE 2 342 TOTAL STAFF BASE 1 608 1 610 1 041 *SET BASE BLACK SOUTH FEMALE SOUTH AFRICANS AFRICANS *Science, engineering and technology -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

Pharmaceuticals in the Environment
Pharmaceuticals in the Environment As a responsible healthcare company, we are committed to the health and safety of our society and planet. We make it a priority to effectively manage the risks associated with pharmaceuticals in the environment (PIE). 1 How do Pharmaceutical Products get into the Environment? Pharmaceuticals enter the environmental mainly as AstraZeneca supports actions based on scientific a result of patient use, where they can pass through evidence to address the challenges presented by PIE. our bodies and into waterways. Drug manufacture and These include: the improper disposal of unused medicines also add • Assessing the environmental risk of our medicines to the trace levels of pharmaceuticals in rivers, lakes, to support drug marketing authorisations soils, and, sometimes, drinking water. AstraZeneca recognises that, even in such low concentrations, • Actively managing the environmental risks resulting the risks associated with Pharmaceuticals in the from our manufacturing Environment (PIE) should be determined, minimised • Supporting industry and government efforts to and managed. improve medicine disposal programs and education • Co-sponsoring research to fill scientific gaps 88% to understand and mitigate the risks of PIE. as a result of patient use To learn more about the risks associated with PIE and what we are doing to manage them please click here. 2% attributed to waste Pharmaceuticals from production in the environment 10% from unused medicines that people don’t dispose of properly 2 Societal Concerns about PIE Trace amounts of pharmaceuticals have been detected in the environment for more than 20 years. As environmental monitoring expands and the methods for measuring pharmaceuticals in the environment become more sophisticated and detection limits get better, the geographical scope and number of pharmaceuticals measured in the environment will grow. -

Monoclonals 5/10/06 11:51 Page 17
Monoclonals 5/10/06 11:51 Page 17 Therapeutics MONOCLONALS the billion dollar molecules of the future In 1975, two British scientists thought of creating a mouse antibody that could be replicated or ‘cloned’ to produce identical copies. Identical copies with identical modes of action had the potential to be a drug.They had launched what was to be one of biotechnology’s best ideas. If the pair could mimic the immune system’s ‘seek and destroy’ capability by pre-designing antibodies for all manner of disease targets, it should be possible to block or activate cellular activity to order. It was an elegant concept opening up a raft of therapeutic possibilities, particularly in the area of cancer. Here it might be possible to attach a chemotherapy drug to an antibody.The antibody’s specificity for a particular disease target would guide the chemo drug only to the cancerous cells and not the healthy ones.The concept, therefore, was excellent, but the use of mice monoclonals had drawbacks.The human body did not like antibodies from mice anymore than it liked the flu virus, so the antibodies themselves triggered an immune response and were destroyed.This ‘immunogenicity’ issue was to occupy researchers for another 20 or so years before it became possible to craft a monoclonal antibody (mAb) that would be acceptable to the human immune system. fter rather a long period in the desert, untreatable diseases. A further six lead drugs are in By Dr Martin Wiles monoclonal antibody drugs are once the final stages of clinical trials and approximately and Patrik A again generating a wave of excitement. -
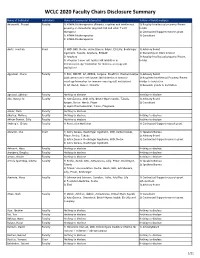
WCLC 2020 Faculty Chairs Disclosure Summary
WCLC 2020 Faculty Chairs Disclosure Summary Name of Individual Individual's Name of Commercial Interest(s) Nature of Relationship(s) Adusumilli, Prasad FacultyRole(s) in Activity 1) ATARA Biotherapeutics (Patents, royalties and intellectual 1) Royalty/Intellectual property/Patent property on mesothelin-targeted CAR and other T-cell holder therapies) 2) Contracted/Support research grant 2) ATARA Biotherapeutics 3) Consultant 3) ATARA Biotherapeutics Aerts, Joachim Chair 1) MSD, BMS, Roche, Astra-Zeneca, BAyer, Eli-Lilly, Boehringer 1) Advisory Board Ingelheim, Takeda, Amphera, BIOCAD 2) Ownership or Stock interest 2) Amphera 3) Royalty/Intellectual property/Patent 3) allogenic tumor cell lysate/JAK inhibition in holder immunooncology/ biomarker for immuno-oncology (all institution) Aggarwal, Charu Faculty 1) BMS, ROCHE, AZ, MERCK, Celgene, BluePrint, Diachaii Sankyo 1) Advisory Board 2)Allogenic tumor cell lysate/JAK inhibition in immuno- 2) Royalties/Intellectual Property/Patent oncology/biomarker for immuno-oncology (all institution) Holder to institution 3) AZ, Merck, Xencor, Novartis 3) Research grants to institution Agrawal, Abhinav Faculty Nothing to disclose Nothing to disclose Ahn, Myung-Ju Faculty 1) AstraZeneca, MSD, Lilly, Bristol-Myers Squibb, Takeda, 1) Advisory Board Amgen, Roche, Merck, Pfizer 2) Consultant 2) Alpha Pharmaceutical, Yuhan, Progenere Aisner, Dara Faculty Nothing to disclose Akerley, Wallace Faculty Nothing to disclose Nothing to disclose Akhtar-Danesh, Gilly Faculty Nothing to disclose Nothing to disclose -

6Th Annual Translational Medicine Conference City Hotel, Derry/Londonderry, Northern Ireland 25Th - 26Th Sept 2014
6th Annual Translational Medicine Conference ‘Personalising Health and Care’ Date: 25th and 26th September 2014 | Venue: City Hotel, Derry/Londonderry, N.Ireland, UK 1 6th Annual Translational Medicine Conference City Hotel, Derry/Londonderry, Northern Ireland 25th - 26th Sept 2014 Conference Sponsors 2 6th Annual Translational Medicine Conference City Hotel, Derry/Londonderry, Northern Ireland 25th - 26th Sept 2014 Contents Page Conference Programme .................................................................................... 4 Biographies .......................................................................................................... 6 - 9 Delegate List ........................................................................................................ 10 - 12 Bio-Entrepreneur Programme........................................................................ 13 About C-TRIC ....................................................................................................... 14 - 15 Abstracts Poster Presentations................................................................. 16 - 42 Abstracts Oral Presentations................................................................. 43 Abstracts Poster List................................................................. 44 3 6th Annual Translational Medicine Conference City Hotel, Derry/Londonderry, Northern Ireland 25th - 26th Sept 2014 ‘Personalising Health and Care’ - Conference Programme Day One Thursday 25th Sept 1:00 pm Registration and Tea/Coffee 4.30 pm Oral -

Alderley Park Development Framework
Alderley Park Development Framework Consultation Draft - January 2015 0i 2 Contents 1 Introduction 2 The Site 3 Planning Policy 4 Design Guidance 5 Indicative Masterplan 6 Planning Applications 7 Summary and Next Steps Appendices 3 1 Introduction Alderley Park, a research and development site The Taskforce commissioned a study to establish the new vision for the site. It allocates Alderley Park as an renowned for the discovery and development of potential future demand from the life science sector, for ‘opportunity site’, seeking to promote and encourage the innovative new medicines, is a key part of the North the world class laboratory and office space on site1. That development of the Life Science Park whilst recognising West Life Science Ecosystem. Opening more than 40 study indicated that whilst there would not by any means that there is likely to be a need for a degree of flexibility years ago, the site has a rich heritage of important be an instant demand for all the site’s facilities, with an regarding land uses to deliver, grow and sustain the Life advancements in medical treatments, including a appropriate business model, there is potential to build on Science Park vision. number of anti-cancer treatments. As the lead centre the BioHub concept, repurposing the site to offer facilities Until the adoption of the CELPS, the current development for cancer research, Alderley Park currently houses which complement existing life science resources across plan for the area remains the Macclesfield Borough the global Advanced Lead Discovery Centre, and its the region, such that Alderley Park can continue to be a Local Plan.