Revista TPP 35(5)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Grapevine Virus Diseases: Economic Impact and Current Advances in Viral Prospection and Management1
1/22 ISSN 0100-2945 http://dx.doi.org/10.1590/0100-29452017411 GRAPEVINE VIRUS DISEASES: ECONOMIC IMPACT AND CURRENT ADVANCES IN VIRAL PROSPECTION AND MANAGEMENT1 MARCOS FERNANDO BASSO2, THOR VINÍCIUS MArtins FAJARDO3, PASQUALE SALDARELLI4 ABSTRACT-Grapevine (Vitis spp.) is a major vegetative propagated fruit crop with high socioeconomic importance worldwide. It is susceptible to several graft-transmitted agents that cause several diseases and substantial crop losses, reducing fruit quality and plant vigor, and shorten the longevity of vines. The vegetative propagation and frequent exchanges of propagative material among countries contribute to spread these pathogens, favoring the emergence of complex diseases. Its perennial life cycle further accelerates the mixing and introduction of several viral agents into a single plant. Currently, approximately 65 viruses belonging to different families have been reported infecting grapevines, but not all cause economically relevant diseases. The grapevine leafroll, rugose wood complex, leaf degeneration and fleck diseases are the four main disorders having worldwide economic importance. In addition, new viral species and strains have been identified and associated with economically important constraints to grape production. In Brazilian vineyards, eighteen viruses, three viroids and two virus-like diseases had already their occurrence reported and were molecularly characterized. Here, we review the current knowledge of these viruses, report advances in their diagnosis and prospection of new species, and give indications about the management of the associated grapevine diseases. Index terms: Vegetative propagation, plant viruses, crop losses, berry quality, next-generation sequencing. VIROSES EM VIDEIRAS: IMPACTO ECONÔMICO E RECENTES AVANÇOS NA PROSPECÇÃO DE VÍRUS E MANEJO DAS DOENÇAS DE ORIGEM VIRAL RESUMO-A videira (Vitis spp.) é propagada vegetativamente e considerada uma das principais culturas frutíferas por sua importância socioeconômica mundial. -
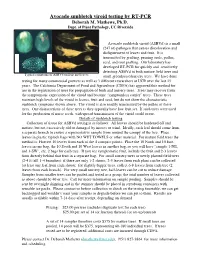
Avocado Sunblotch Viroid Testing by RT-PCR Deborah M
Avocado sunblotch viroid testing by RT-PCR Deborah M. Mathews, Ph.D. Dept. of Plant Pathology, UC Riverside Avocado sunblotch viroid (ASBVd) is a small (247 nt) pathogen that causes discoloration and disfigurement of leaves and fruit. It is transmitted by grafting, pruning tools, pollen, seed, and root grafting. Our laboratory has developed RT-PCR for quickly and sensitively detecting ASBVd in both mature field trees and Typical symptoms of ASBVd on fruit and leaves small greenhouse/nursery trees. We have done testing for many commercial growers as well as 3 different researchers at UCR over the last 15 years. The California Department of Food and Agriculture (CDFA) has approved this method for use in the registration of trees for propagation of buds and nursery trees. Trees may recover from the symptomatic expression of the viroid and become “symptomless carrier” trees. These trees maintain high levels of the viroid in leaves, fruit and seed, but do not show the characteristic sunblotch symptoms shown above. The viroid is also readily transmitted by the pollen of these trees. One characteristic of these trees is they typically have low fruit set. If such trees were used for the production of nurse seeds, widespread transmission of the viroid could occur. Details of sunblotch testing Collection of tissue for ASBVd testing is as follows: All leaves should be hardened off and mature, but not excessively old or damaged by insects or wind. Ideally, each leaf should come from a separate branch to ensure a representative sample from around the canopy of the tree. Place leaves in plastic ziplock bags with NO WET TOWELS or other material. -

Hops – a Guide for New Growers 2017
Hops – a guide for new growers 2017 growers new – a guide for Hops Hops a guide for new growers FIRST EDITION 2017 first edition 2017 Author: Kevin Dodds www.dpi.nsw.gov.au Hops a guide for new growers Kevin Dodds Development Officer – Temperate Fruits NSW Department of Primary industries ©NSW Department of Primary Industries 2017 Published by NSW Department of Primary Industries, a part of NSW Department of Industry, Skills and Regional Development You may copy, distribute, display, download and otherwise freely deal with this publication for any purpose, provided that you attribute NSW Department of Industry, Skills and Regional Development as the owner. However, you must obtain permission if you wish to charge others for access to the publication (other than at cost); include the publication advertising or a product for sale; modify the publication; or republish the publication on a website. You may freely link to the publication on a departmental website. First published March 2017 ISBN print: 978‑1‑76058‑007‑0 web: 978‑1‑76058‑008‑7 Always read the label Users of agricultural chemical products must always read the Job number 14293 label and any permit before using the product and strictly comply with the directions on the label and the conditions of Author any permit. Users are not absolved from any compliance with Kevin Dodds, Development Officer Temperate Fruits the directions on the label or the conditions of the permit NSW Department of Primary Industries by reason of any statement made or omitted to be made in 64 Fitzroy Street TUMUT NSW 2720 this publication. -

Detection of Avocado Sunblotch Viroid and Estimation of Infection Among Accessions in the National Germplasm Collection for Avocado
Proc. Fla. State Hort. Soc. 109:235-237. 1996. DETECTION OF AVOCADO SUNBLOTCH VIROID AND ESTIMATION OF INFECTION AMONG ACCESSIONS IN THE NATIONAL GERMPLASM COLLECTION FOR AVOCADO Catherine M. Running and Raymond J. Schnell National Germplasm Repository U. S. Department of Agriculture Agricultural Research Service 13601 Old Cutler Road, Miami, FL 33158 David N. Kuhn Florida International University Dept. of Biological Sciences Miami, FL 33199 Additional index words. Persea Americana, RT-PCR, viroid, DNA, RNA, sequence variation, germplasm. ABSTRACT Reverse transcription-polymerase chain reaction (RTPCR) was used to determine the incidence of infection by avocado sunblotch viroid (ASBVd) in the germplasm collection at the National Germplasm Repository at Miami (NGR-Mia). Of the 429 avocado plants growing at the repository, 81 (18.9%) are infected with the viroid. The 429 plants represent 237 accessions. There are multiple plants of some accessions and for 42 accessions (17%) every plant is infected with the viroid. There was no apparent relationship between host race and the incidence of infection. Symptoms of sunblotch disease on avocado (Persea Americana Mill.) manifest as a general decline of tree vigor with sunken, yellow areas on the fruit that lessen its marketability. The disease was first described as infectious, rather than physiological, by Home and Parker (1931). The infectious agent has since been determined to be an RNA viroid known as Avocado Sunblotch Viroid (ASBVd) (Dale and Allen, 1979; Thomas and Mohammed, 1979). The viroid is transmitted by budding and grafting, including natural root grafting (Home etal, 1941; Whitsell, 1952), seed (Wallace and Drake, 1962), and pollen (Desjardins et al., 1979). -

THE MOLECULAR CHARACTERIZATION of the VIRUS and VIRUS-LIKE AGENTS PRESENT in TA TAO 5 GERMPLASM of PRUNUS PERSICA Diana Marini Clemson University, [email protected]
Clemson University TigerPrints All Dissertations Dissertations 12-2007 THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA Diana Marini Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Part of the Agronomy and Crop Sciences Commons Recommended Citation Marini, Diana, "THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA " (2007). All Dissertations. 143. https://tigerprints.clemson.edu/all_dissertations/143 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Plant and Environmental Sciences by Diana Beatriz Marini December 2007 Accepted by: Dr. Simon W. Scott, Committee Chair Dr. N. Dwight Camper Dr. Steven N. Jeffers Dr. Gregory L. Reighard ABSTRACT Peach production in the southeastern United States is limited by late spring freezes. Ta Tao 5 germplasm, used either as an interstem or by chip bud inoculation, has been shown to delay bloom and avoid the effects of these late freezes. The growth modification is graft transmissible and the germplasm has been found to be infected with ACLSV, APruV-3, and PLMVd. Using a combination of PCR, cloning, and sequencing techniques, a molecular characterization of the three graft-transmissible agents present in Ta Tao 5 has been completed. -

Replication of Avocado Sunblotch Viroid in the Cyanobacterium Nostoc Sp. PCC 7120
atholog P y & nt a M Latifi et al., J Plant Pathol Microbiol 2016, 7:4 l i P c f r o o DOI: 10.4172/2157-7471.1000341 b l i Journal of a o l n o r g u y o J ISSN: 2157-7471 Plant Pathology & Microbiology Research Article Open Access Replication of Avocado Sunblotch Viroid in the Cyanobacterium Nostoc Sp. PCC 7120 Amel Latifi1*, Christophe Bernard1, Laura da Silva2, Yannick Andéol3, Amine Elleuch4, Véronique Risoul1, Jacques Vergne2 and Marie-Christine Maurel2 1Aix Marseille University, CNRS, UMR Chemistry Laboratory Bacterial 7283. 31 Chemin Joseph Aiguier 13009 Marseille Cedex 20, France 2Institute of Systematics, Evolution, Biodiversity ( ISyEB ), CNRS, MNHN , UPMC, EPHE, UPMC Sorbonne University, 57 rue Cuvier, PO Box 50, F-75005 Paris, France 3Team Enzymology of RNA, UR6, UPMC Sorbonne University, 75252 Paris Cedex 05 France 4Laboratory of Plant Biotechnology, Faculty of Sciences of Sfax, University of Sfax, BP 1171, 3000 Sfax, Tunisia Abstract Viroids are small infectious RNA molecules that replicate in plants via RNA-RNA replication processes. The molecular mechanism responsible for this replication has attracted great interest, and studies on this topic have yielded interesting biological findings on the processes in which RNA is involved. Viroids belonging to the Avsunviroidae family replicate in the chloroplasts of infected hosts. It has by now been established that chloroplasts and cyanobacteria share a common have ancestor. In view of this phylogenetic relationship, we investigated whether a member of the Avsunviroidae family could be replicated in a cyanobacterium. The results obtained here show that Avocado Sunblotch Viroid (ASBVd) RNA is able to replicate in the filamentous cyanobacterium Nostoc PCC 7120. -

Recognizing Avocado Sunblotch Disease
RECOGNIZING AVOCADO SUNBLOTCH DISEASE 1J. A. Dodds, 1D Mathews, 2M. L. Arpaia and 3G. W. Witney. 1Dept. Plant Pathology, University of California, Riverside, CA 92521. A. 2Dept. Botany and Plant Sciences, University of California, Riverside, CA 92521. 3California Avocado Commission, 1251 E. Dyer Rd., Suite 210, Santa Ana, CA 92705. Avocado sunblotch is a disease which has been known for over 60 years. Sunblotch is characterized by abnormal tree growth, reduced yield and a high proportion of small or misshapen fruit. Specific symptoms may be observed on fruit, leaves and stems. Fruit may show overall distortion, sometimes with sunken yellow and red depressions on the surface. These fruit are graded as standards and receive lower returns from the packinghouse (Figure A). Foliar symptoms can include a general thinning of B. the canopy, with individual leaves showing bleaching (white patches), variegation (yellow and green patches), and distortion (Figure B, C). Stems may also show discoloration with yellow, white or pink streaks (Figure D), and in extreme cases may be completely chlorotic (yellow/white). Infected trees often appear stunted and somewhat sprawling. It is possible for trees to “recover” from the disease. The “recovered” tree will have no apparent visual disease symptoms but it still carries the viroid. These trees are termed “symptomless” carriers. Such trees typically have very low fruit yield or at times may set heavy crops of small fruit. If symptomless trees are topworked with disease-free material, the topworked material will become infected and can exhibit classical sunblotch symptoms revealing C. the presence of sunblotch. If a symptomless tree is subject to a stress such as fire or is stumped, the regrowth may once again exhibit sunblotch symptoms. -

The Avocado Sunblotch Viroid: an Invisible Foe of Avocado
viruses Review The Avocado Sunblotch Viroid: An Invisible Foe of Avocado José Ramón Saucedo Carabez 1,*, Daniel Téliz Ortiz 2 , Moisés Roberto Vallejo Pérez 3 and Hugo Beltrán Peña 4 1 Departamento de Investigación Aplicada-Driscolls, Libramiento Sur #1620, Jacona 59833, Michoacán, Mexico 2 Postgrado en Fitosanidad-Fitopatología, Colegio de Postgraduados, Km. 36.5, Montecillo, Texcoco 56230, Estado de México, Mexico; [email protected] 3 Consejo Nacional de Ciencia y Tecnología-Universidad Autónoma de San Luis Potosí, Álvaro Obregón #64, San Luis Potosí 78000, San Luis Potosí, Mexico; [email protected] 4 Departamento de Ciencias Naturales y Exactas-Fitopatología, Universidad Autónoma de Occidente UR Los Mochis, Boulevard Macario Gaxiola, Los Mochis 81223, Sinaloa, Mexico; [email protected] * Correspondence: [email protected] Received: 1 May 2019; Accepted: 25 May 2019; Published: 29 May 2019 Abstract: This review collects information about the history of avocado and the economically important disease, avocado sunblotch, caused by the avocado sunblotch viroid (ASBVd). Sunblotch symptoms are variable, but the most common in fruits are irregular sunken areas of white, yellow, or reddish color. On severely affected fruits, the sunken areas may become necrotic. ASBVd (type species Avocado sunblotch viroid, family Avsunviroidae) replicates and accumulates in the chloroplast, and it is the smallest plant pathogen. This pathogen is a circular single-stranded RNA of 246–251 nucleotides. ASBVd has a restricted host range and only few plant species of the family Lauraceae have been confirmed experimentally as additional hosts. The most reliable method to detect ASBVd in the field is to identify symptomatic fruits, complemented in the laboratory with reliable and sensitive molecular techniques to identify infected but asymptomatic trees. -

EPPO Reporting Service
ORGANISATION EUROPEENNE ET MEDITERRANEENNE POUR LA PROTECTION DES PLANTES EUROPEAN AND MEDITERRANEAN PLANT PROTECTION ORGANIZATION EPPO Reporting Service NO. 10 PARIS, 2020-10 General 2020/209 New additions to the EPPO A1 and A2 Lists 2020/210 New data on quarantine pests and pests of the EPPO Alert List 2020/211 New and revised dynamic EPPO datasheets are available in the EPPO Global Database 2020/212 Recommendations from Euphresco projects Pests 2020/213 First report of Spodoptera frugiperda in Jordan 2020/214 Trogoderma granarium does not occur in Spain 2020/215 First report of Scirtothrips dorsalis in Mexico 2020/216 First report of Scirtothrips dorsalis in Brazil 2020/217 Scirtothrips dorsalis occurs in Colombia 2020/218 Update on the situation of Megaplatypus mutatus in Italy 2020/219 Update on the situation of Anoplophora chinensis in Croatia 2020/220 Update on the situation of Anoplophora chinensis in Italy 2020/221 Update on the situation of Anoplophora glabripennis in Italy Diseases 2020/222 Eradication of thousand canker disease in disease in Toscana (Italy) 2020/223 First report of tomato brown rugose fruit virus in the Czech Republic 2020/224 Update on the situation of tomato brown rugose fruit virus in Greece 2020/225 Update on the situation of tomato brown rugose fruit virus in the Netherlands 2020/226 New finding of ‘Candidatus Liberibacter solanacearum’ in Estonia 2020/227 Haplotypes and vectors of ‘Candidatus Liberibacter solanacearum’ in Scotland (United Kingdom) 2020/228 First report of wheat blast in Zambia and in -

Viroid-2018 Book of Abstracts
Viroid-2018 Viroid-2018 International Conference on Viroids and Viroid-Like RNAs 5-7 July 2018, Valencia, Spain Polytechnic City of Innovation, Universitat Politècnica de València Avenida de los Naranjos, 46022 Valencia, Spain http://www.ibmcp.upv.es/viroid-2018/ [email protected] Book of Abstracts Organizer: José-Antonio Daròs Instituto de Biología Molecular y Celular de Plantas (IBMCP) CSIC-Universitat Politècnica de Valencia Avenida de los Naranjos, 46022Valencia, Spain [email protected] http://personales.upv.es/jadaros/ Updated version, 3 July 2018 1 Viroid-2018 INDEX Supporting institutions and contributing companies ……………… 3 Conference venue …………………………………….……….……… 4 Conference program ………………………………………………… 6 List of participants ………………………………………...………… 12 Abstracts, oral presentations …………….………….……….……… 16 Abstracts, poster presentations …………….………….……….…… 47 Index of authors ……………………………………………………… 70 Notes …………………………..……………………….……………… 72 2 Viroid-2018 SUPPORTING INSTITUTIONS Grants from Ministerio de Ciencia, Innovación y Universidades (Spain): BIO2014-54269-R, BIO2017-83184-R and BIO2017-91865-EXP Grant from Generalitat Valenciana: AORG/2018/114 CONTRIBUTING COMPANIES Biotest Diagnosticos S.L., http://www.biotestdiagnosticos.es/ Cultek, http://www.cultek.com/ Durviz, http://durviz.com/ Épica, http://epicasl.com/ Fisher Scientific, http://www.fishersci.com GenoChem, http://geno-chem.com/ IDT, http://www.idtdna.com/pages NZYTech, http://www.nzytech.com/ Werfen, http://es.werfen.com/ VWR, http://www.vwr.com/ 3 Viroid-2018 CONFERENCE VENUE Campus Universitat Politècnica de Valencia, corner between Avenida de los Naranjos and Calle Ingeniero Fausto Elio, Access J, Ciudad Politécnica de la Innovación (CPI), Red Prism. 4 Viroid-2018 5 Viroid-2018 CONFERENCE PROGRAM Thursday, July 5th 09:00-10:00. Registration at CPI Red Prism 10:00-10:20. Opening Chairman: Robert A. -

Determination of the Dominant Variants of Hop Stunt Viroid in Two Different Cachexia Isolates from North and South of Iran
J. Agr. Sci. Tech. (2016) Vol. 18: 1431-1440 Determination of the Dominant Variants of Hop Stunt Viroid in Two Different Cachexia Isolates from North and South of Iran S. N. Banihashemian 1, S. M. Bani Hashemian 2*, and S. M. Ashkan 1 ABSTRACT Citrus plants are hosts of several viroid species, among which, pathogenic variants of Hop Stunt Viroid (HSVd) induce citrus cachexia disease. Stunting, chlorosis, gumming of the bark, stem pitting and decline are symptoms of cachexia in mandarins and their hybrids as susceptible hosts. Based on the pathogenic properties on citrus, HSVd variants are divided in two distinct groups: those that are symptomless on sensitive citrus host species and those that induce cachexia disease. In this study, two cachexia isolates were selected and biological indexing was performed in a controlled temperature greenhouse (40ºC day and 28ºC night) using Etrog citron (Citrus medica ) grafted on Rough lemon ( C. jhambiri ), as a common indicator for citrus viroids. The plants were inoculated with the inocula from a severe symptomatic tree of a newly declining orchard of Jiroft, Kerman province and a mild symptomatic tree from Mazandaran province. Presence of HSVd was confirmed with sPAGE, Hybridization by DIG-labeled probes and RT-PCR using specific primers of HSVd . Primary and secondary structures of the isolates were studied. The consensus sequence of RT-PCR amplicons of the severe isolate (JX430796) presented 97% identity with the reference sequence of a IIb variant of HSVd (AF213501) and an Iranian isolate of the viroid (GQ923783) deposited in the gene bank. The mild isolate (JX430798) presented 100% homology with the HSVd-IIc variant previously reported from Iran (GQ923784). -

First Report of Hop Stunt Viroid Infecting Vitis Gigas, V. Flexuosa and Ampelopsis Heterophylla
Australasian Plant Disease Notes (2018) 13:3 https://doi.org/10.1007/s13314-017-0287-9 First report of Hop stunt viroid infecting Vitis gigas, V. flexuosa and Ampelopsis heterophylla Thor Vinícius Martins Fajardo1 & Marcelo Eiras2 & Osmar Nickel1 Received: 10 November 2017 /Accepted: 27 December 2017 # Australasian Plant Pathology Society Inc. 2018 Abstract Hop stunt viroid (HSVd) is one of the most common viroids that infect grapevine (Vitis spp.) worldwide. Sixteen sequences of the HSVd genome were obtained from infected grapevines in Brazil by next generation sequencing (NGS). Multiple alignments of the sequences showed nucleotide identities ranging from 94.6% to 100%. This is the first report of HSVd infecting two wild grape species and Ampelopsis heterophylla. These HSVd isolates along with others from V. vinifera and V. labrusca were phylogenetically analyzed. Keywords Next generation sequencing . HSVd . Incidence . Genetic variability . Vitis Grapevine (Vitis spp.) is a globally important fruit crop con- hosts including trees, shrubs and herbaceous plants, with the sidering its socioeconomic importance and cultivated area. majority of isolates to date identified from citrus species, Among graft-transmissible grapevine pathogens, viruses and followed by grapevine and stone fruits (Prunus spp.). HSVd viroids can reduce plant vigor, yield, productivity and fruit causes disease symptoms, such as hop stunt, dappled fruits in quality. Losses are especially significant in mixed infections plum and peach trees, and citrus cachexia (Jo et al. 2017). The (Basso et al. 2017). Viroids are naked, non-protein-coding, viroid can be transmitted vegetatively, mechanically, or via small (246–401 nt) covalently closed, circular single- grape seeds (Wan Chow Wah and Symons 1999).