Symptomatic Plant Viroid Infections in Phytopathogenic Fungi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Grapevine Virus Diseases: Economic Impact and Current Advances in Viral Prospection and Management1
1/22 ISSN 0100-2945 http://dx.doi.org/10.1590/0100-29452017411 GRAPEVINE VIRUS DISEASES: ECONOMIC IMPACT AND CURRENT ADVANCES IN VIRAL PROSPECTION AND MANAGEMENT1 MARCOS FERNANDO BASSO2, THOR VINÍCIUS MArtins FAJARDO3, PASQUALE SALDARELLI4 ABSTRACT-Grapevine (Vitis spp.) is a major vegetative propagated fruit crop with high socioeconomic importance worldwide. It is susceptible to several graft-transmitted agents that cause several diseases and substantial crop losses, reducing fruit quality and plant vigor, and shorten the longevity of vines. The vegetative propagation and frequent exchanges of propagative material among countries contribute to spread these pathogens, favoring the emergence of complex diseases. Its perennial life cycle further accelerates the mixing and introduction of several viral agents into a single plant. Currently, approximately 65 viruses belonging to different families have been reported infecting grapevines, but not all cause economically relevant diseases. The grapevine leafroll, rugose wood complex, leaf degeneration and fleck diseases are the four main disorders having worldwide economic importance. In addition, new viral species and strains have been identified and associated with economically important constraints to grape production. In Brazilian vineyards, eighteen viruses, three viroids and two virus-like diseases had already their occurrence reported and were molecularly characterized. Here, we review the current knowledge of these viruses, report advances in their diagnosis and prospection of new species, and give indications about the management of the associated grapevine diseases. Index terms: Vegetative propagation, plant viruses, crop losses, berry quality, next-generation sequencing. VIROSES EM VIDEIRAS: IMPACTO ECONÔMICO E RECENTES AVANÇOS NA PROSPECÇÃO DE VÍRUS E MANEJO DAS DOENÇAS DE ORIGEM VIRAL RESUMO-A videira (Vitis spp.) é propagada vegetativamente e considerada uma das principais culturas frutíferas por sua importância socioeconômica mundial. -

Changes to Virus Taxonomy 2004
Arch Virol (2005) 150: 189–198 DOI 10.1007/s00705-004-0429-1 Changes to virus taxonomy 2004 M. A. Mayo (ICTV Secretary) Scottish Crop Research Institute, Invergowrie, Dundee, U.K. Received July 30, 2004; accepted September 25, 2004 Published online November 10, 2004 c Springer-Verlag 2004 This note presents a compilation of recent changes to virus taxonomy decided by voting by the ICTV membership following recommendations from the ICTV Executive Committee. The changes are presented in the Table as decisions promoted by the Subcommittees of the EC and are grouped according to the major hosts of the viruses involved. These new taxa will be presented in more detail in the 8th ICTV Report scheduled to be published near the end of 2004 (Fauquet et al., 2004). Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., and Ball, L.A. (eds) (2004). Virus Taxonomy, VIIIth Report of the ICTV. Elsevier/Academic Press, London, pp. 1258. Recent changes to virus taxonomy Viruses of vertebrates Family Arenaviridae • Designate Cupixi virus as a species in the genus Arenavirus • Designate Bear Canyon virus as a species in the genus Arenavirus • Designate Allpahuayo virus as a species in the genus Arenavirus Family Birnaviridae • Assign Blotched snakehead virus as an unassigned species in family Birnaviridae Family Circoviridae • Create a new genus (Anellovirus) with Torque teno virus as type species Family Coronaviridae • Recognize a new species Severe acute respiratory syndrome coronavirus in the genus Coro- navirus, family Coronaviridae, order Nidovirales -

The Variability of Hop Latent Viroid As Induced Upon Heat Treatment
Virology 287, 349–358 (2001) doi:10.1006/viro.2001.1044, available online at http://www.idealibrary.com on View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector The Variability of Hop Latent Viroid as Induced upon Heat Treatment Jaroslav Matousˇek,* Josef Patzak,† Lidmila Orctova´,* Jo¨rg Schubert,‡ Luka´sˇ Vrba,* Gerhard Steger,§ and Detlev Riesner§,1 *Department of Molecular Genetics, Institute of Plant Molecular Biology Czech Academy of Sciences, Branisˇovska´31, 37005 Cˇ eske´Bude˘jovice, Czech Republic; †Department of Virology, Institute of Hop Research and Breeding, Kadanˇska´2525, 438 46 Zˇatec, Czech Republic; ‡Federal Centre for Breeding Research, Institute for Resistance Research and Pathogen Diagnostics, Theodor-Roemer-Weg 4, 06449 Aschersleben, Germany; and §Institute of Physical Biology, Heinrich-Heine Universita¨t Du¨sseldorf, Universita¨tsstraße 1, D-40225 Du¨sseldorf, Germany Received March 28, 2001; returned to author for revision March 30, 2001; accepted June 11, 2001; published online August 2, 2001 We have previously shown that heat treatment of hop plants infected by hop latent viroid (HLVd) reduces viroid levels. Here we investigate whether such heat treatment leads to the accumulation of sequence variability in HLVd. We observed a negligible level of mutated variants in HLVd under standard cultivation conditions. In contrast, the heat treatment of hop led to HLVd degradation and, simultaneously, to a significant increase in sequence variations, as judged from temperature gradient–gel electrophoresis analysis and cDNA library screening by DNA heteroduplex analysis. Thirty-one cDNA clones (9.8%) were identified as deviating forms. -

(12) United States Patent (10) Patent No.: US 9,096,585 B2 Shaw Et Al
US009096585B2 (12) United States Patent (10) Patent No.: US 9,096,585 B2 Shaw et al. (45) Date of Patent: Aug. 4, 2015 (54) ANTIVIRAL COMPOUNDS AND USES (2013.01); C07D 413/10 (2013.01); C07D THEREOF 413/12 (2013.01); A61 K 3 1/5377 (2013.01); A61 K3I/55 (2013.01) (75) Inventors: Megan Shaw, New York, NY (US); (58) Field of Classification Search Hans-Heinrich Hoffmann, New York, CPC. A61K 31/4245; A61K31/5377; A61K31/55 NY (US); Adolfo Garcia-Sastre, New USPC .................................... 514/364, 217.1, 236.2 York, NY (US); Peter Palese, Leonia, NJ See application file for complete search history. US (US) (56) References Cited (73) Assignee: Icahn School of Medicine at Mount Sinai, New York, NY (US) U.S. PATENT DOCUMENTS 6,384,064 B2 5, 2002 Camden (*) Notice: Subject to any disclaimer, the term of this 8,278,342 B2 10/2012 Ricciardi patent is extended or adjusted under 35 Continued U.S.C. 154(b) by 210 days. (Continued) (21) Appl. No.: 13? 700,049 FOREIGN PATENT DOCUMENTS y x- - - 9 (22) PCT Filed1C Mavay 31,51, 2011 WO WO 2009,136979 A2 11/2009 OTHER PUBLICATIONS (86). PCT No.: PCT/US2011/038515 Chu et al. "Analysis of the endocytic pathway mediating the infec S371 (c)(1), tious entry of mosquito-borne flavivirus West Nile into Aedes (2), (4) Date: Feb. 14, 2013 albopictus mosquito (C6/36) cells.” Virology, 2006, vol. 349, pp. 463-475. (87) PCT Pub. No.: WO2011/150413 (Continued) PCT Pub. Date: Dec. 1, 2011 Primary Examiner — Shengjun Wang (65) Prior Publication Data (74) Attorney, Agent, or Firm — Jones Day US 2013/O137678A1 May 30, 2013 (57) ABSTRACT O O Described herein are Compounds, compositions comprising Related U.S. -

Rapid Identification of Known and New RNA Viruses from Animal Tissues
Rapid Identification of Known and New RNA Viruses from Animal Tissues Joseph G. Victoria1,2*, Amit Kapoor1,2, Kent Dupuis3, David P. Schnurr3, Eric L. Delwart1,2 1 Department of Molecular Virology, Blood Systems Research Institute, San Francisco, California, United States of America, 2 Department of Laboratory Medicine, University of California, San Francisco, California, United States of America, 3 Viral and Rickettsial Disease Laboratory, Division of Communicable Disease Control, California State Department of Public Health, Richmond, California, United States of America Abstract Viral surveillance programs or diagnostic labs occasionally obtain infectious samples that fail to be typed by available cell culture, serological, or nucleic acid tests. Five such samples, originating from insect pools, skunk brain, human feces and sewer effluent, collected between 1955 and 1980, resulted in pathology when inoculated into suckling mice. In this study, sequence-independent amplification of partially purified viral nucleic acids and small scale shotgun sequencing was used on mouse brain and muscle tissues. A single viral agent was identified in each sample. For each virus, between 16% to 57% of the viral genome was acquired by sequencing only 42–108 plasmid inserts. Viruses derived from human feces or sewer effluent belonged to the Picornaviridae family and showed between 80% to 91% amino acid identities to known picornaviruses. The complete polyprotein sequence of one virus showed strong similarity to a simian picornavirus sequence in the provisional Sapelovirus genus. Insects and skunk derived viral sequences exhibited amino acid identities ranging from 25% to 98% to the segmented genomes of viruses within the Reoviridae family. Two isolates were highly divergent: one is potentially a new species within the orthoreovirus genus, and the other is a new species within the orbivirus genus. -
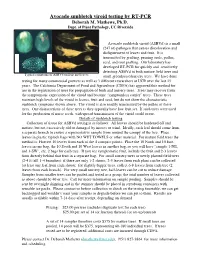
Avocado Sunblotch Viroid Testing by RT-PCR Deborah M
Avocado sunblotch viroid testing by RT-PCR Deborah M. Mathews, Ph.D. Dept. of Plant Pathology, UC Riverside Avocado sunblotch viroid (ASBVd) is a small (247 nt) pathogen that causes discoloration and disfigurement of leaves and fruit. It is transmitted by grafting, pruning tools, pollen, seed, and root grafting. Our laboratory has developed RT-PCR for quickly and sensitively detecting ASBVd in both mature field trees and Typical symptoms of ASBVd on fruit and leaves small greenhouse/nursery trees. We have done testing for many commercial growers as well as 3 different researchers at UCR over the last 15 years. The California Department of Food and Agriculture (CDFA) has approved this method for use in the registration of trees for propagation of buds and nursery trees. Trees may recover from the symptomatic expression of the viroid and become “symptomless carrier” trees. These trees maintain high levels of the viroid in leaves, fruit and seed, but do not show the characteristic sunblotch symptoms shown above. The viroid is also readily transmitted by the pollen of these trees. One characteristic of these trees is they typically have low fruit set. If such trees were used for the production of nurse seeds, widespread transmission of the viroid could occur. Details of sunblotch testing Collection of tissue for ASBVd testing is as follows: All leaves should be hardened off and mature, but not excessively old or damaged by insects or wind. Ideally, each leaf should come from a separate branch to ensure a representative sample from around the canopy of the tree. Place leaves in plastic ziplock bags with NO WET TOWELS or other material. -

The Viruses of Wild Pigeon Droppings
The Viruses of Wild Pigeon Droppings Tung Gia Phan1,2, Nguyen Phung Vo1,3,A´ kos Boros4,Pe´ter Pankovics4,Ga´bor Reuter4, Olive T. W. Li6, Chunling Wang5, Xutao Deng1, Leo L. M. Poon6, Eric Delwart1,2* 1 Blood Systems Research Institute, San Francisco, California, United States of America, 2 Department of Laboratory Medicine, University of California San Francisco, San Francisco, California, United States of America, 3 Pharmacology Department, School of Pharmacy, Ho Chi Minh City University of Medicine and Pharmacy, Ho Chi Minh, Vietnam, 4 Regional Laboratory of Virology, National Reference Laboratory of Gastroenteric Viruses, A´ NTSZ Regional Institute of State Public Health Service, Pe´cs, Hungary, 5 Stanford Genome Technology Center, Stanford, California, United States of America, 6 Centre of Influenza Research and School of Public Health, University of Hong Kong, Hong Kong SAR Abstract Birds are frequent sources of emerging human infectious diseases. Viral particles were enriched from the feces of 51 wild urban pigeons (Columba livia) from Hong Kong and Hungary, their nucleic acids randomly amplified and then sequenced. We identified sequences from known and novel species from the viral families Circoviridae, Parvoviridae, Picornaviridae, Reoviridae, Adenovirus, Astroviridae, and Caliciviridae (listed in decreasing number of reads), as well as plant and insect viruses likely originating from consumed food. The near full genome of a new species of a proposed parvovirus genus provisionally called Aviparvovirus contained an unusually long middle ORF showing weak similarity to an ORF of unknown function from a fowl adenovirus. Picornaviruses found in both Asia and Europe that are distantly related to the turkey megrivirus and contained a highly divergent 2A1 region were named mesiviruses. -

Hops – a Guide for New Growers 2017
Hops – a guide for new growers 2017 growers new – a guide for Hops Hops a guide for new growers FIRST EDITION 2017 first edition 2017 Author: Kevin Dodds www.dpi.nsw.gov.au Hops a guide for new growers Kevin Dodds Development Officer – Temperate Fruits NSW Department of Primary industries ©NSW Department of Primary Industries 2017 Published by NSW Department of Primary Industries, a part of NSW Department of Industry, Skills and Regional Development You may copy, distribute, display, download and otherwise freely deal with this publication for any purpose, provided that you attribute NSW Department of Industry, Skills and Regional Development as the owner. However, you must obtain permission if you wish to charge others for access to the publication (other than at cost); include the publication advertising or a product for sale; modify the publication; or republish the publication on a website. You may freely link to the publication on a departmental website. First published March 2017 ISBN print: 978‑1‑76058‑007‑0 web: 978‑1‑76058‑008‑7 Always read the label Users of agricultural chemical products must always read the Job number 14293 label and any permit before using the product and strictly comply with the directions on the label and the conditions of Author any permit. Users are not absolved from any compliance with Kevin Dodds, Development Officer Temperate Fruits the directions on the label or the conditions of the permit NSW Department of Primary Industries by reason of any statement made or omitted to be made in 64 Fitzroy Street TUMUT NSW 2720 this publication. -

Virus, Viroids and Mycoplasma
By: Dr. Bibha Kumari Dept. of Zoology Magadh Mahila College, Patna Email: [email protected] Virus •The viruses are non-cellular organisms. • They, in fact, have an inert crystalline structure outside the living cell. • Once they infect a cell, they take over the machinery of the host cell to replicate themselves, killing the host. •Pasteur. D.J. Ivanowsky (1892) gave the name virus. • It means venom or poisonous fluid. • According to his research, certain microbes caused the mosaic disease of tobacco. •These organisms were smaller than bacteria because they passed through bacteria-proof filters. • M.W. Beijerinek (1898) demonstrated that the extract of the infected plants of tobacco could cause infection in healthy plants. • He named the fluid as Contagium vivum fluidum (infectious living fluid). •W.M. Stanley (1935) discovered that viruses could be crystallized. These virus crystals are composed largely of proteins. •They are inert outside their specific host cell. Viruses are nothing but obligate parasites. Genetic Material of Viruses: •In addition to proteins, viruses also contain genetic material, that could be either RNA or DNA. • No virus contains both RNA and DNA. A virus is a nucleoprotein and the genetic material is infectious. •Speaking in strictly general terms, viruses infecting plants have single- stranded RNA. • On the other hand, viruses that infect animals have either single or double-stranded RNA or they might have double-stranded DNA •Bacterial viruses or bacteriophages usually have a double-stranded DNA structure. By bacteriophages, we mean viruses that infect the bacteria. • The protein coat, capsid made of small subunits (capsomeres) protects the nucleic acid. -

Molecular Variability of Apple Hammerhead Viroid from Italian
Virus Research 270 (2019) 197644 Contents lists available at ScienceDirect Virus Research journal homepage: www.elsevier.com/locate/virusres Short communication Molecular variability of apple hammerhead viroid from Italian apple varieties supports the relevance in vivo of its branched conformation T stabilized by a kissing loop interaction Michela Chiumentia, Beatriz Navarroa, Pasquale Veneritob, Francesco Civitac, ⁎ ⁎ Angelantonio Minafraa, , Francesco Di Serioa, a Istituto per la Protezione Sostenibile delle Piante (CNR), Bari, Italy b Centro di Ricerca, Sperimentazione e Formazione in Agricoltura “Basile Caramia”, Locorotondo, Italy c SINAGRI – Università degli Studi di Bari “Aldo Moro”, Bari, Italy ARTICLE INFO ABSTRACT Keywords: In the absence of protein-coding ability, viroid RNAs rely on direct interactions with host factors for their Hammerhead infectivity. RNA structural elements are likely involved in these interactions. Therefore, preservation of a Non-Coding RNA structural element, despite the sequence variability existing between the variants of a viroid population, is Sequence variability considered a solid evidence of its relevant role in vivo. In this study, apple hammerhead viroid (AHVd) was first Secondary structure identified in the two apple cultivars ‘Mela Rosa Guadagno’ (MRG) and ‘Agostinella’ (AG), which are cultivated Co-Variation since long in Southern Italy, thus providing the first solid evidence of its presence in this country. Then, the Stem-Loop natural variability of AHVd viroid populations infecting MRG and AG was studied. The sequence variants from the two Italian isolates shared only 82.1–87.7% sequence identity with those reported previously from other geographic areas, thus providing the possibility of exploring the impact of this sequence divergence on the proposed secondary structure. -

Detection of Avocado Sunblotch Viroid and Estimation of Infection Among Accessions in the National Germplasm Collection for Avocado
Proc. Fla. State Hort. Soc. 109:235-237. 1996. DETECTION OF AVOCADO SUNBLOTCH VIROID AND ESTIMATION OF INFECTION AMONG ACCESSIONS IN THE NATIONAL GERMPLASM COLLECTION FOR AVOCADO Catherine M. Running and Raymond J. Schnell National Germplasm Repository U. S. Department of Agriculture Agricultural Research Service 13601 Old Cutler Road, Miami, FL 33158 David N. Kuhn Florida International University Dept. of Biological Sciences Miami, FL 33199 Additional index words. Persea Americana, RT-PCR, viroid, DNA, RNA, sequence variation, germplasm. ABSTRACT Reverse transcription-polymerase chain reaction (RTPCR) was used to determine the incidence of infection by avocado sunblotch viroid (ASBVd) in the germplasm collection at the National Germplasm Repository at Miami (NGR-Mia). Of the 429 avocado plants growing at the repository, 81 (18.9%) are infected with the viroid. The 429 plants represent 237 accessions. There are multiple plants of some accessions and for 42 accessions (17%) every plant is infected with the viroid. There was no apparent relationship between host race and the incidence of infection. Symptoms of sunblotch disease on avocado (Persea Americana Mill.) manifest as a general decline of tree vigor with sunken, yellow areas on the fruit that lessen its marketability. The disease was first described as infectious, rather than physiological, by Home and Parker (1931). The infectious agent has since been determined to be an RNA viroid known as Avocado Sunblotch Viroid (ASBVd) (Dale and Allen, 1979; Thomas and Mohammed, 1979). The viroid is transmitted by budding and grafting, including natural root grafting (Home etal, 1941; Whitsell, 1952), seed (Wallace and Drake, 1962), and pollen (Desjardins et al., 1979). -

THE MOLECULAR CHARACTERIZATION of the VIRUS and VIRUS-LIKE AGENTS PRESENT in TA TAO 5 GERMPLASM of PRUNUS PERSICA Diana Marini Clemson University, [email protected]
Clemson University TigerPrints All Dissertations Dissertations 12-2007 THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA Diana Marini Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Part of the Agronomy and Crop Sciences Commons Recommended Citation Marini, Diana, "THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA " (2007). All Dissertations. 143. https://tigerprints.clemson.edu/all_dissertations/143 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Plant and Environmental Sciences by Diana Beatriz Marini December 2007 Accepted by: Dr. Simon W. Scott, Committee Chair Dr. N. Dwight Camper Dr. Steven N. Jeffers Dr. Gregory L. Reighard ABSTRACT Peach production in the southeastern United States is limited by late spring freezes. Ta Tao 5 germplasm, used either as an interstem or by chip bud inoculation, has been shown to delay bloom and avoid the effects of these late freezes. The growth modification is graft transmissible and the germplasm has been found to be infected with ACLSV, APruV-3, and PLMVd. Using a combination of PCR, cloning, and sequencing techniques, a molecular characterization of the three graft-transmissible agents present in Ta Tao 5 has been completed.