AVOCADO SUNBLOTCH VIROID (Asbvd) in CELL AND
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of Bioactive Molecules in Palm Oil Pulp
139 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 79, 2020 The Italian Association of Chemical Engineering Online at www.cetjournal.it Guest Editors: Enrico Bardone, Antonio Marzocchella, Marco Bravi Copyright © 2020, AIDIC Servizi S.r.l. DOI: 10.3303/CET2079024 ISBN 978-88-95608-77-8; ISSN 2283-9216 Characterization of Bioactive Molecules in Palm Oil Pulp (Elaeis Guineensis Jacq.) a b* Selvin Antonio Saravia Maldonado , Ismael Montero Fernández , Bernardo de c d e Morais Linhares , Jhunior Abrahan Marcia Fuentes , Ricardo Santos Alemán , Vanny Perpetua Ferrazf a Faculty of Earth Sciences and Conservation, National University of Agriculture, Highway to Dulce Nombre de Culmi, km 215, Neighborhood El Espino, Catacamas-Olancho, Honduras. bUniversity of Extremadura. Department of Organic and Inorganic Chemistry. Polytechnic School, University Avenue s/n, Cáceres, Spain. cFederal Institute of Roraima. Campus Boa Vista, Av. Glaycon de Paiva, 2496 - Pricumã, Boa Vista - RR, 69303-340. d Faculty of Technological Sciences, National University of Agriculture, Highway to Dulce Nombre de Culmi, km 215, Neighborhood El Espino, Catacamas-Olancho, Honduras. eDepartment of Food Science, Louisiana State University, U.S. fChromatography Laboratory, Universidade Federal de Minas Gerais, Belo Horizonte-MG-Brazil. [email protected] Oil palm (Elaeis guineensis Jacq.) is a crop that has great economic potential, since its productive potential is extremely high. It is grown in several countries in Asia, Africa and South America, with the main purpose of producing biodiesel raw material for the cosmetics and food industry. In Brazil, its cultivation is concentrated in only a few small regions, distributed mainly in the Northeast and North of the country. -

Study of Hydrogenation Derived Renewable Diesel As a Renewable Fuel Option in North America
Study of Hydrogenation Derived Renewable Diesel as a Renewable Fuel Option in North America Final Report Natural Resources Canada 580 Booth Street Ottawa, Ontario K1A 0E4 For additional information, please contact: Natalie Lambert Project Manager, Energy Telephone: 514 562-8651 Email: [email protected] March 30,2012 Experts in environment and natural resource economics ■stHSfesa ■ 825, Raoul-Jobin, Quebec (Quebec) Canada GIN 1S6 1097, St-Alexandre, Suite 201, Montreal (Quebec) Canada H2Z IPS www.ecoressources.com • [email protected] Study of Hydrogenation Derived Renewable Diesel as a Renewable Fuel Option in North America - Final Report Executive Summary As of 2011, 27 national governments and 29 state/province governments have implemented policies that mandate the use of a minimum amount of renewable alternatives to diesel, including Europe, six South American countries, six Asian countries, Canada, the United States, Costa Rica and the Dominican Republic. On June 29, 2011, the government of Canada registered regulations amending the Renewable Fuels Regulations which were then published on July 20, 201 11. These amendments stated that the coming into force date of the 2% requirement of renewable content in diesel and heating oil would be July 1st, 2011. Under the Renewable Fuels Regulations, both ester-based biodiesel and hydrogenation-derived renewable diesel (HDRD) are admissible as renewable content that can be used to meet the requirements of the Regulations. While biodiesel is the most widely available diesel fuel alternative, there has been increasing interest by the regulated parties in using HDRD to meet the requirements, even though HDRD is currently only produced in Europe, Southeast Asia and the United States 23. -

Current Knowledge on Interspecific Hybrid Palm Oils As Food and Food
foods Review Current Knowledge on Interspecific Hybrid Palm Oils as Food and Food Ingredient Massimo Mozzon , Roberta Foligni * and Cinzia Mannozzi * Department of Agricultural, Food and Environmental Sciences, Università Politecnica delle Marche, Via Brecce Bianche 10, 60131 Ancona, Italy; m.mozzon@staff.univpm.it * Correspondence: r.foligni@staff.univpm.it (R.F.); c.mannozzi@staff.univpm.it (C.M.); Tel.: +39-071-220-4010 (R.F.); +39-071-220-4014 (C.M.) Received: 6 April 2020; Accepted: 10 May 2020; Published: 14 May 2020 Abstract: The consumers’ opinion concerning conventional palm (Elaeis guineensis) oil is negatively affected by environmental and nutritional issues. However, oils extracted from drupes of interspecific hybrids Elaeis oleifera E. guineensis are getting more and more interest, due to their chemical and × nutritional properties. Unsaturated fatty acids (oleic and linoleic) are the most abundant constituents (60%–80% of total fatty acids) of hybrid palm oil (HPO) and are mainly acylated in position sn-2 of the glycerol backbone. Carotenes and tocotrienols are the most interesting components of the unsaponifiable matter, even if their amount in crude oils varies greatly. The Codex Committee on Fats and Oils recently provided HPO the “dignity” of codified fat substance for human consumption and defined the physical and chemical parameters for genuine crude oils. However, only few researches have been conducted to date on the functional and technological properties of HPO, thus limiting its utilization in food industry. Recent studies on the nutritional effects of HPO softened the initial enthusiasm about the “tropical equivalent of olive oil”, suggesting that the overconsumption of HPO in the most-consumed processed foods should be carefully monitored. -
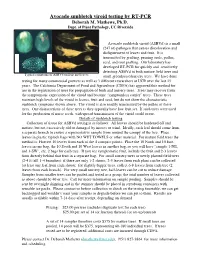
Avocado Sunblotch Viroid Testing by RT-PCR Deborah M
Avocado sunblotch viroid testing by RT-PCR Deborah M. Mathews, Ph.D. Dept. of Plant Pathology, UC Riverside Avocado sunblotch viroid (ASBVd) is a small (247 nt) pathogen that causes discoloration and disfigurement of leaves and fruit. It is transmitted by grafting, pruning tools, pollen, seed, and root grafting. Our laboratory has developed RT-PCR for quickly and sensitively detecting ASBVd in both mature field trees and Typical symptoms of ASBVd on fruit and leaves small greenhouse/nursery trees. We have done testing for many commercial growers as well as 3 different researchers at UCR over the last 15 years. The California Department of Food and Agriculture (CDFA) has approved this method for use in the registration of trees for propagation of buds and nursery trees. Trees may recover from the symptomatic expression of the viroid and become “symptomless carrier” trees. These trees maintain high levels of the viroid in leaves, fruit and seed, but do not show the characteristic sunblotch symptoms shown above. The viroid is also readily transmitted by the pollen of these trees. One characteristic of these trees is they typically have low fruit set. If such trees were used for the production of nurse seeds, widespread transmission of the viroid could occur. Details of sunblotch testing Collection of tissue for ASBVd testing is as follows: All leaves should be hardened off and mature, but not excessively old or damaged by insects or wind. Ideally, each leaf should come from a separate branch to ensure a representative sample from around the canopy of the tree. Place leaves in plastic ziplock bags with NO WET TOWELS or other material. -

Detection of Avocado Sunblotch Viroid and Estimation of Infection Among Accessions in the National Germplasm Collection for Avocado
Proc. Fla. State Hort. Soc. 109:235-237. 1996. DETECTION OF AVOCADO SUNBLOTCH VIROID AND ESTIMATION OF INFECTION AMONG ACCESSIONS IN THE NATIONAL GERMPLASM COLLECTION FOR AVOCADO Catherine M. Running and Raymond J. Schnell National Germplasm Repository U. S. Department of Agriculture Agricultural Research Service 13601 Old Cutler Road, Miami, FL 33158 David N. Kuhn Florida International University Dept. of Biological Sciences Miami, FL 33199 Additional index words. Persea Americana, RT-PCR, viroid, DNA, RNA, sequence variation, germplasm. ABSTRACT Reverse transcription-polymerase chain reaction (RTPCR) was used to determine the incidence of infection by avocado sunblotch viroid (ASBVd) in the germplasm collection at the National Germplasm Repository at Miami (NGR-Mia). Of the 429 avocado plants growing at the repository, 81 (18.9%) are infected with the viroid. The 429 plants represent 237 accessions. There are multiple plants of some accessions and for 42 accessions (17%) every plant is infected with the viroid. There was no apparent relationship between host race and the incidence of infection. Symptoms of sunblotch disease on avocado (Persea Americana Mill.) manifest as a general decline of tree vigor with sunken, yellow areas on the fruit that lessen its marketability. The disease was first described as infectious, rather than physiological, by Home and Parker (1931). The infectious agent has since been determined to be an RNA viroid known as Avocado Sunblotch Viroid (ASBVd) (Dale and Allen, 1979; Thomas and Mohammed, 1979). The viroid is transmitted by budding and grafting, including natural root grafting (Home etal, 1941; Whitsell, 1952), seed (Wallace and Drake, 1962), and pollen (Desjardins et al., 1979). -

THE MOLECULAR CHARACTERIZATION of the VIRUS and VIRUS-LIKE AGENTS PRESENT in TA TAO 5 GERMPLASM of PRUNUS PERSICA Diana Marini Clemson University, [email protected]
Clemson University TigerPrints All Dissertations Dissertations 12-2007 THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA Diana Marini Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Part of the Agronomy and Crop Sciences Commons Recommended Citation Marini, Diana, "THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA " (2007). All Dissertations. 143. https://tigerprints.clemson.edu/all_dissertations/143 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. THE MOLECULAR CHARACTERIZATION OF THE VIRUS AND VIRUS-LIKE AGENTS PRESENT IN TA TAO 5 GERMPLASM OF PRUNUS PERSICA A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Plant and Environmental Sciences by Diana Beatriz Marini December 2007 Accepted by: Dr. Simon W. Scott, Committee Chair Dr. N. Dwight Camper Dr. Steven N. Jeffers Dr. Gregory L. Reighard ABSTRACT Peach production in the southeastern United States is limited by late spring freezes. Ta Tao 5 germplasm, used either as an interstem or by chip bud inoculation, has been shown to delay bloom and avoid the effects of these late freezes. The growth modification is graft transmissible and the germplasm has been found to be infected with ACLSV, APruV-3, and PLMVd. Using a combination of PCR, cloning, and sequencing techniques, a molecular characterization of the three graft-transmissible agents present in Ta Tao 5 has been completed. -

Replication of Avocado Sunblotch Viroid in the Cyanobacterium Nostoc Sp. PCC 7120
atholog P y & nt a M Latifi et al., J Plant Pathol Microbiol 2016, 7:4 l i P c f r o o DOI: 10.4172/2157-7471.1000341 b l i Journal of a o l n o r g u y o J ISSN: 2157-7471 Plant Pathology & Microbiology Research Article Open Access Replication of Avocado Sunblotch Viroid in the Cyanobacterium Nostoc Sp. PCC 7120 Amel Latifi1*, Christophe Bernard1, Laura da Silva2, Yannick Andéol3, Amine Elleuch4, Véronique Risoul1, Jacques Vergne2 and Marie-Christine Maurel2 1Aix Marseille University, CNRS, UMR Chemistry Laboratory Bacterial 7283. 31 Chemin Joseph Aiguier 13009 Marseille Cedex 20, France 2Institute of Systematics, Evolution, Biodiversity ( ISyEB ), CNRS, MNHN , UPMC, EPHE, UPMC Sorbonne University, 57 rue Cuvier, PO Box 50, F-75005 Paris, France 3Team Enzymology of RNA, UR6, UPMC Sorbonne University, 75252 Paris Cedex 05 France 4Laboratory of Plant Biotechnology, Faculty of Sciences of Sfax, University of Sfax, BP 1171, 3000 Sfax, Tunisia Abstract Viroids are small infectious RNA molecules that replicate in plants via RNA-RNA replication processes. The molecular mechanism responsible for this replication has attracted great interest, and studies on this topic have yielded interesting biological findings on the processes in which RNA is involved. Viroids belonging to the Avsunviroidae family replicate in the chloroplasts of infected hosts. It has by now been established that chloroplasts and cyanobacteria share a common have ancestor. In view of this phylogenetic relationship, we investigated whether a member of the Avsunviroidae family could be replicated in a cyanobacterium. The results obtained here show that Avocado Sunblotch Viroid (ASBVd) RNA is able to replicate in the filamentous cyanobacterium Nostoc PCC 7120. -

Recognizing Avocado Sunblotch Disease
RECOGNIZING AVOCADO SUNBLOTCH DISEASE 1J. A. Dodds, 1D Mathews, 2M. L. Arpaia and 3G. W. Witney. 1Dept. Plant Pathology, University of California, Riverside, CA 92521. A. 2Dept. Botany and Plant Sciences, University of California, Riverside, CA 92521. 3California Avocado Commission, 1251 E. Dyer Rd., Suite 210, Santa Ana, CA 92705. Avocado sunblotch is a disease which has been known for over 60 years. Sunblotch is characterized by abnormal tree growth, reduced yield and a high proportion of small or misshapen fruit. Specific symptoms may be observed on fruit, leaves and stems. Fruit may show overall distortion, sometimes with sunken yellow and red depressions on the surface. These fruit are graded as standards and receive lower returns from the packinghouse (Figure A). Foliar symptoms can include a general thinning of B. the canopy, with individual leaves showing bleaching (white patches), variegation (yellow and green patches), and distortion (Figure B, C). Stems may also show discoloration with yellow, white or pink streaks (Figure D), and in extreme cases may be completely chlorotic (yellow/white). Infected trees often appear stunted and somewhat sprawling. It is possible for trees to “recover” from the disease. The “recovered” tree will have no apparent visual disease symptoms but it still carries the viroid. These trees are termed “symptomless” carriers. Such trees typically have very low fruit yield or at times may set heavy crops of small fruit. If symptomless trees are topworked with disease-free material, the topworked material will become infected and can exhibit classical sunblotch symptoms revealing C. the presence of sunblotch. If a symptomless tree is subject to a stress such as fire or is stumped, the regrowth may once again exhibit sunblotch symptoms. -

Synthesis of High-Titer Alka(E)Nes in Yarrowia Lipolytica Is Enabled by a Discovered Mechanism
ARTICLE https://doi.org/10.1038/s41467-020-19995-0 OPEN Synthesis of high-titer alka(e)nes in Yarrowia lipolytica is enabled by a discovered mechanism Jingbo Li 1, Yongshuo Ma 1, Nian Liu 1, Bekir E. Eser 2, Zheng Guo 2, Peter Ruhdal Jensen3 & ✉ Gregory Stephanopoulos 1 Alka(e)nes are ideal fuel components for aviation, long-distance transport, and shipping. They are typically derived from fossil fuels and accounting for 24% of difficult-to-eliminate 1234567890():,; greenhouse gas emissions. The synthesis of alka(e)nes in Yarrowia lipolytica from CO2- neutral feedstocks represents an attractive alternative. Here we report that the high-titer synthesis of alka(e)nes in Yarrowia lipolytica harboring a fatty acid photodecarboxylase (CvFAP) is enabled by a discovered pathway. We find that acyl-CoAs, rather than free fatty acids (FFAs), are the preferred substrate for CvFAP. This finding allows us to debottleneck the pathway and optimize fermentation conditions so that we are able to redirect 89% of acyl-CoAs from the synthesis of neutral lipids to alka(e)nes and reach titers of 1.47 g/L from glucose. Two other CO2-derived substrates, wheat straw and acetate, are also demonstrated to be effective in producing alka(e)nes. Overall, our technology could advance net-zero emissions by providing CO2-neutral and energy-dense liquid biofuels. 1 Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02142, USA. 2 Department of Engineering, Aarhus University, Gustav Wieds Vej 10, Aarhus 8000, Denmark. 3 National Food Institute, Technical University of Denmark, Kongens Lyngby 2800, Denmark. -

The Avocado Sunblotch Viroid: an Invisible Foe of Avocado
viruses Review The Avocado Sunblotch Viroid: An Invisible Foe of Avocado José Ramón Saucedo Carabez 1,*, Daniel Téliz Ortiz 2 , Moisés Roberto Vallejo Pérez 3 and Hugo Beltrán Peña 4 1 Departamento de Investigación Aplicada-Driscolls, Libramiento Sur #1620, Jacona 59833, Michoacán, Mexico 2 Postgrado en Fitosanidad-Fitopatología, Colegio de Postgraduados, Km. 36.5, Montecillo, Texcoco 56230, Estado de México, Mexico; [email protected] 3 Consejo Nacional de Ciencia y Tecnología-Universidad Autónoma de San Luis Potosí, Álvaro Obregón #64, San Luis Potosí 78000, San Luis Potosí, Mexico; [email protected] 4 Departamento de Ciencias Naturales y Exactas-Fitopatología, Universidad Autónoma de Occidente UR Los Mochis, Boulevard Macario Gaxiola, Los Mochis 81223, Sinaloa, Mexico; [email protected] * Correspondence: [email protected] Received: 1 May 2019; Accepted: 25 May 2019; Published: 29 May 2019 Abstract: This review collects information about the history of avocado and the economically important disease, avocado sunblotch, caused by the avocado sunblotch viroid (ASBVd). Sunblotch symptoms are variable, but the most common in fruits are irregular sunken areas of white, yellow, or reddish color. On severely affected fruits, the sunken areas may become necrotic. ASBVd (type species Avocado sunblotch viroid, family Avsunviroidae) replicates and accumulates in the chloroplast, and it is the smallest plant pathogen. This pathogen is a circular single-stranded RNA of 246–251 nucleotides. ASBVd has a restricted host range and only few plant species of the family Lauraceae have been confirmed experimentally as additional hosts. The most reliable method to detect ASBVd in the field is to identify symptomatic fruits, complemented in the laboratory with reliable and sensitive molecular techniques to identify infected but asymptomatic trees. -

April 9, 2020 Replacement of Primex Brand Hydrogenated Vegetable
April 9, 2020 Replacement of Primex Brand Hydrogenated Vegetable Shortening In June of 2018 the FDA banned the use of trans fats in human foods. Due to the ban, Envigo was no longer able to source Primex brand hydrogenated vegetable oil (HVO) containing trans fats. Beginning on April 11th of 2018, Primex was replaced with an USP grade HVO made to a food-grade standard that has a similar texture and fatty acid profile (see table). Manufacturing tests revealed no appreciable differences in physical qualities of finished diets. Although diet numbers did not change, you may have noticed an updated diet title and ingredient description on the diet datasheet. Depending on your research goals and desire for relevance to human diets, you may wish to use a source of HVO without trans fats such as Crisco. Crisco is a proprietary HVO with minimal trans fats (see table). Envigo also offers several popular obesity inducing diets with alternate fat sources like lard or milkfat that may be suitable for your research. Contact a nutritionist to discuss alternate options. Comparison of the fatty acid profile of Primex, Envigo Teklad’s Replacement HVO and Crisco. Fatty Acids, % Primex HVO1 Replacement HVO2 Crisco3 Trans fatty acids 23.9 - 36.1 26 - 35.6 0.6 Saturated fatty acids 25.3 - 27.1 22.6 - 29.3 25.8 Monounsaturated fatty acids 25.3 - 33.3 24.8 - 32.6 18.7 Polyunsaturated fatty acids 5.6 - 9.0 7.1 - 9.4 49.5 16:0 palmitic acid 14.0 - 17.4 11.0 - 14.8 16.9 18:0 stearic acid 9.1 - 11.5 10.7 - 14.2 9.6 18:1 n9T elaidic acid 22.2 - 34.7 24.4 - 32.6 0 18:1 n9C oleic acid 16.6 - 26.5 17.2 - 25.7 18.1 18:1 n7C vaccenic acid 2.2 - 2.4 2.0 - 2.2 1.2 18:1 other cis isomers 6.3 - 7.8 6.1 - 6.7 0 18:2 n6 linoleic acid 5.8 - 9.1 7.0 - 9.2 44.8 18:2 other trans isomers 3.2 - 4.0 3.5 - 5.0 0.5 18:3 n3 linolenic acid 0.3 - 0.5 0.1 - 0.3 6.1 19:0 nonadecanoic acid 0.6 - 0.7 0.4 - 0.7 0 20:0 arachidic acid 0.3 - 0.4 0.4 0.4 1Range for Primex HVO represents the average ± 1 standard deviation (soybean and cottonseed or palm oil; n = 4). -

Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel Over Novel N-Doped Activated Carbon Supported Pt Nanoparticles
energies Article Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles Wei Jin 1, Laura Pastor-Pérez 1,2, Juan J. Villora-Pico 2, Mercedes M. Pastor-Blas 2, Antonio Sepúlveda-Escribano 2, Sai Gu 1, Nikolaos D. Charisiou 3, Kyriakos Papageridis 3, Maria A. Goula 3,* and Tomas R. Reina 1,* 1 Chemical & Process Engineering Department, University of Surrey, Guildford GU2 7XH, UK; [email protected] (W.J.); [email protected] (L.P.-P.); [email protected] (S.G.) 2 Laboratorio de Materiales Avanzados, Departamento de Química Inorgánica Instituto Universitario de Materiales de Alicante, Universidad de Alicante, 03690 Alicante, Spain; [email protected] (J.J.V.-P.); [email protected] (M.M.P.-B.); [email protected] (A.S.-E.) 3 Department of Chemical Engineering, University of Western Macedonia, 50100 Kozani, Greece; [email protected] (N.D.C.); [email protected] (K.P.) * Correspondence: [email protected] (M.A.G.); [email protected] (T.R.R.); Tel.: +44-148-368-6597 (T.R.R.) Received: 12 November 2019; Accepted: 20 December 2019; Published: 26 December 2019 Abstract: Bio-hydrogenated diesel (BHD), derived from vegetable oil via hydrotreating technology, is a promising alternative transportation fuel to replace nonsustainable petroleum diesel. In this work, a novel Pt-based catalyst supported on N-doped activated carbon prepared from polypyrrole as the nitrogen source (Pt/N-AC) was developed and applied in the palm oil deoxygenation process to produce BHD in a fixed bed reactor system.