Araneae, Salticidae, Pelleninae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Open PDF Printout of This File
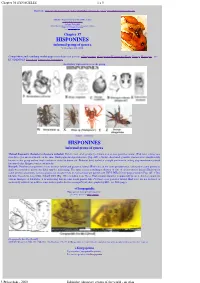
Chapter 36 SYNAGELES 1 z 5 Main links: Title page Introduction & guides INDEXES_chapters_&_genera Open PDF printout of this file Salticidae (Araneae) genera of the world - an atlas (unfinished manuscript) by Jerzy Prószy ński Professor Emeritus, Museum and Institute of Zoology, Polish Academy of Sciences, ul. Wilcza 63, 00-679 Warsaw, POLAND e-mail: [email protected] Chapter 37 HISPONINES informal group of genera Version June 3rd, 2020. symbol of the supragroup HISPONINES Composition and searching on this page (including fossil genera): +Gorgopsidis : +Gorgopsina Hermotimus Hispo Jerzego Massagris - see EUODENINES Tomobella Tomocyrba Tomomingi Exemplary representatives of the group Source - see below HISPONINES informal group of genera Mutual diagnostic characters of genera included . Differs from other groups by minute size of eyes posterior lateral (IInd row), sitting very closely to eyes anterior lateral, on the same black pigmented protuberance (Fig. 49J1). On the other hand, genitalic characters are insufficiently known in this group and no final conclusion could be drawn yet. Embolus bent, coiled or straight and minute, sitting atop membranous distal haematodocha. Epigyne varies extensively. Remark. Peculiar arrangement of eyes anterior lateral and posterior lateral (IInd row) on the same protuberance, followed in some genera by shallow constriction across eye field, may be misleading. The same concerns striking reduction of size of eyes posterior lateral (IInd row) in some genera classified to various groups, for instance eyes in Lystrocteissa are paired with HYLLINES[?] like palp structure (Figs 14L, 17G), likewise Tomobella fotsy Szüts, Scharff 2009 (Fig. 49I) resembles some Neon . That somatic character is apparently prone to develop parallel in various lineages of Salticidae, it is interesting that in some fossil genera (like Prolinus ) eyes posterior lateral (IInd row) are not reduced, or moderately reduced (as in Hisponinae amber spider shown on magnificent photographs by Hill - see Title page). -

Arachnids (Excluding Acarina and Pseudoscorpionida) of the Wichita Mountains Wildlife Refuge, Oklahoma
OCCASIONAL PAPERS THE MUSEUM TEXAS TECH UNIVERSITY NUMBER 67 5 SEPTEMBER 1980 ARACHNIDS (EXCLUDING ACARINA AND PSEUDOSCORPIONIDA) OF THE WICHITA MOUNTAINS WILDLIFE REFUGE, OKLAHOMA JAMES C. COKENDOLPHER AND FRANK D. BRYCE The Wichita Mountains are located in eastern Greer, southern Kiowa, and northwestern Comanche counties in Oklahoma. Since their formation more than 300 million years ago, these rugged mountains have been fragmented and weathered, until today the highest peak (Mount Pinchot) stands only 756 meters above sea level (Tyler, 1977). The mountains are composed predominantly of granite and gabbro. Forests of oak, elm, and walnut border most waterways, while at elevations from 153 to 427 meters prair ies are the predominant vegetation type. A more detailed sum mary of the climatic and biotic features of the Wichitas has been presented by Blair and Hubbell (1938). A large tract of land in the eastern range of the Wichita Moun tains (now northeastern Comanche County) was set aside as the Wichita National Forest by President McKinley during 1901. In 1905, President Theodore Roosevelt created a game preserve on those lands managed by the Forest Service. Since 1935, this pre serve has been known as the Wichita Mountains Wildlife Refuge. Numerous papers on Oklahoma spiders have been published (Bailey and Chada, 1968; Bailey et al., 1968; Banks et al, 1932; Branson, 1958, 1959, 1966, 1968; Branson and Drew, 1972; Gro- thaus, 1968; Harrel, 1962, 1965; Horner, 1975; Rogers and Horner, 1977), but only a single, comprehensive work (Banks et al., 1932) exists covering all arachnid orders in the state. Further additions and annotations to the arachnid fauna of Oklahoma can be found 2 OCCASIONAL PAPERS MUSEUM TEXAS TECH UNIVERSITY in recent revisionary studies. -

Diversity of Simonid Spiders (Araneae: Salticidae: Salticinae) in India
IJBI 2 (2), (DECEMBER 2020) 247-276 International Journal of Biological Innovations Available online: http://ijbi.org.in | http://www.gesa.org.in/journals.php DOI: https://doi.org/10.46505/IJBI.2020.2223 Review Article E-ISSN: 2582-1032 DIVERSITY OF SIMONID SPIDERS (ARANEAE: SALTICIDAE: SALTICINAE) IN INDIA Rajendra Singh1*, Garima Singh2, Bindra Bihari Singh3 1Department of Zoology, Deendayal Upadhyay University of Gorakhpur (U.P.), India 2Department of Zoology, University of Rajasthan, Jaipur (Rajasthan), India 3Department of Agricultural Entomology, Janta Mahavidyalaya, Ajitmal, Auraiya (U.P.), India *Corresponding author: [email protected] Received: 01.09.2020 Accepted: 30.09.2020 Published: 09.10.2020 Abstract: Distribution of spiders belonging to 4 tribes of clade Simonida (Salticinae: Salticidae: Araneae) reported in India is dealt. The tribe Aelurillini (7 genera, 27 species) is represented in 16 states and in 2 union territories, Euophryini (10 genera, 16 species) in 14 states and in 4 union territories, Leptorchestini (2 genera, 3 species) only in 2 union territories, Plexippini (22 genera, 73 species) in all states except Mizoram and in 3 union territories, and Salticini (3 genera, 11 species) in 15 states and in 4 union terrioties. West Bengal harbours maximum number of species, followed by Tamil Nadu and Maharashtra. Out of 129 species of the spiders listed, 70 species (54.3%) are endemic to India. Keywords: Aelurillini, Euophryini, India, Leptorchestini, Plexippini, Salticidae, Simonida. INTRODUCTION Hisponinae, Lyssomaninae, Onomastinae, Spiders are chelicerate arthropods belonging to Salticinae and Spartaeinae. Out of all the order Araneae of class Arachnida. Till to date subfamilies, Salticinae comprises 93.7% of the 48,804 described species under 4,180 genera and species (5818 species, 576 genera, including few 128 families (WSC, 2020). -

Local and Regional Influences on Arthropod Community
LOCAL AND REGIONAL INFLUENCES ON ARTHROPOD COMMUNITY STRUCTURE AND SPECIES COMPOSITION ON METROSIDEROS POLYMORPHA IN THE HAWAIIAN ISLANDS A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI'I IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY (ECOLOGY, EVOLUTION AND CONSERVATION BIOLOGy) AUGUST 2004 By Daniel S. Gruner Dissertation Committee: Andrew D. Taylor, Chairperson John J. Ewel David Foote Leonard H. Freed Robert A. Kinzie Daniel Blaine © Copyright 2004 by Daniel Stephen Gruner All Rights Reserved. 111 DEDICATION This dissertation is dedicated to all the Hawaiian arthropods who gave their lives for the advancement ofscience and conservation. IV ACKNOWLEDGEMENTS Fellowship support was provided through the Science to Achieve Results program of the U.S. Environmental Protection Agency, and training grants from the John D. and Catherine T. MacArthur Foundation and the National Science Foundation (DGE-9355055 & DUE-9979656) to the Ecology, Evolution and Conservation Biology (EECB) Program of the University of Hawai'i at Manoa. I was also supported by research assistantships through the U.S. Department of Agriculture (A.D. Taylor) and the Water Resources Research Center (RA. Kay). I am grateful for scholarships from the Watson T. Yoshimoto Foundation and the ARCS Foundation, and research grants from the EECB Program, Sigma Xi, the Hawai'i Audubon Society, the David and Lucille Packard Foundation (through the Secretariat for Conservation Biology), and the NSF Doctoral Dissertation Improvement Grant program (DEB-0073055). The Environmental Leadership Program provided important training, funds, and community, and I am fortunate to be involved with this network. -

Book of Abstracts
FINAL PROGRAM & ABSTRACTS PROGRAM OVERVIEW (click the day) SUNDAY 08 MONDAY 09 TUESDAY 10 PROGRAM OVERVIEW (click the day) WEDNESDAY 11 THURSDAY 12 FRIDAY 13 31st European Congress of Arachnology Organisers: Hungarian Ecological Society and the Centre for Agricultural Research, Hungarian Academy of Sciences in co-operation with the community of Hungarian arachnologists Co-organising partners: Apor Vilmos Catholic College & European Society of Arachnology 8–13 July, 2018 Vác, Hungary Budapest, 2018 (version 24/VII) Edited by László Mezőfi and Éva Szita Organising Committee Ferenc Samu – chair Csaba Szinetár – co-chair György Dudás Róbert Gallé László Mezőfi Zsolt Szabó Éva Szita Tamás Szűts Natalija Vukaljovic Scientific committee Ferenc Samu co-ordinator Tamás Szűts co-ordinator Dimitar Dimitrov Marco Isaia Simona Kralj Fišer Wolfgang Nentwig Stano Pekár Gabriele Uhl Supporting Committee Zsuzsa Libor, AVKF rector – chair Ervin Balázs, director MTA ATK Zoltán Botta-Dukát, president MÖTE András Füri, director DINP Jenő Kontschán, director PPI, MTA ATK Yuri Marusik, director Russian Party Helpers Erika Botos, János Eichardt, Dániel Erdélyi, Katinka Feketéné Battyáni, Dávid Fülöp, Péter Kovács, Katalin Lehoczki, Teréz Márkus, Gábor Merza, Szilvia Mezőfi, Zsuzsanna Pál, András Rákóczi, Zsolt Szabó, Luca Török, Tamás Török, Violetta Varga, János Vígh The logo The 31st ECA logo, designed by Éva Szita, depicts the uloborid spider Hyptiotes paradoxus perching on the signal thread of its reduced orb-web. The typical triangular orb is framed by -

Jerzego, a New Hisponine Jumping Spider from Borneo (Araneae: Salticidae)
Zootaxa 3852 (5): 569–578 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3852.5.5 http://zoobank.org/urn:lsid:zoobank.org:pub:85C58188-05CE-4EED-A371-8AFE0E8148C8 Jerzego, a new hisponine jumping spider from Borneo (Araneae: Salticidae) WAYNE P. MADDISON1,3 & EDYTA K. PIASCIK2 1Departments of Zoology and Botany and Beaty Biodiversity Museum, University of British Columbia, 6270 University Boulevard, Vancouver, British Columbia, V6T 1Z4, Canada. E-mail: [email protected] 2Department of Zoology, University of British Columbia, 6270 University Boulevard, Vancouver, British Columbia, V6T 1Z4, Canada. E-mail: [email protected] 3Corresponding author Abstract A new genus and species of hisponine jumping spider from Sarawak, Jerzego corticicola Maddison sp. nov. are described, representing one of the few hisponine jumping spiders known from Asia, and the only whose male is known. Although similar to the primarily-Madagascan genus Hispo in having an elongate and flat body, sequences of 28s and 16sND1 genes indicate that Jerzego is most closely related to Massagris and Tomomingi, a result consistent with morphology. Females of Jerzego and other genera of Hisponinae were found to have an unusual double copulatory duct, which appears to be a synapomorphy of the subfamily. Two species are transferred from Hispo, Jerzego bipartitus (Simon) comb. nov. and Jer- zego alboguttatus (Simon) comb. nov. Diagnostic illustrations -

Molecular Insights Into the Phylogenetic Structure of the Spider
MolecularBlackwell Publishing Ltd insights into the phylogenetic structure of the spider genus Theridion (Araneae, Theridiidae) and the origin of the Hawaiian Theridion-like fauna MIQUEL A. ARNEDO, INGI AGNARSSON & ROSEMARY G. GILLESPIE Accepted: 9 March 2007 Arnedo, M. A., Agnarsson, I. & Gillespie, R. G. (2007). Molecular insights into the phylo- doi:10.1111/j.1463-6409.2007.00280.x genetic structure of the spider genus Theridion (Araneae, Theridiidae) and the origin of the Hawaiian Theridion-like fauna. — Zoologica Scripta, 36, 337–352. The Hawaiian happy face spider (Theridion grallator Simon, 1900), named for a remarkable abdominal colour pattern resembling a smiling face, has served as a model organism for under- standing the generation of genetic diversity. Theridion grallator is one of 11 endemic Hawaiian species of the genus reported to date. Asserting the origin of island endemics informs on the evolutionary context of diversification, and how diversity has arisen on the islands. Studies on the genus Theridion in Hawaii, as elsewhere, have long been hampered by its large size (> 600 species) and poor definition. Here we report results of phylogenetic analyses based on DNA sequences of five genes conducted on five diverse species of Hawaiian Theridion, along with the most intensive sampling of Theridiinae analysed to date. Results indicate that the Hawai- ian Islands were colonised by two independent Theridiinae lineages, one of which originated in the Americas. Both lineages have undergone local diversification in the archipelago and have convergently evolved similar bizarre morphs. Our findings confirm para- or polyphyletic status of the largest Theridiinae genera: Theridion, Achaearanea and Chrysso. -

Evolutionary Divergences Mirror Pleistocene Paleodrainages in A
Molecular Phylogenetics and Evolution 144 (2020) 106696 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Evolutionary divergences mirror Pleistocene paleodrainages in a rapidly- T evolving complex of oasis-dwelling jumping spiders (Salticidae, Habronattus tarsalis) ⁎ Marshal Hedina, , Steven Foldia,b, Brendan Rajah-Boyera,c a Dept of Biology, San Diego State University, San Diego, CA 92182, United States b Valley International Prep School, Chatsworth, CA 91311, United States c St. Francis of Assisi Catholic School, Vista, CA 92083, United States ARTICLE INFO ABSTRACT Keywords: We aimed to understand the diversifcation history of jumping spiders in the Habronattus tarsalis species com- Biogeography plex, with particular emphasis on how history in this system might illuminate biogeographic patterns and Ephemeral speciation processes in deserts of the western United States. Desert populations of H. tarsalis are now confned to highly Introgression discontinuous oasis-like habitats, but these habitats would have been periodically more connected during Pleistocene multiple pluvial periods of the Pleistocene. We estimated divergence times using relaxed molecular clock ana- Saline Valley lyses of published transcriptome datasets. Geographic patterns of diversifcation history were assessed using Sexual selection phylogenetic and cluster analyses of original sequence capture, RADSeq and morphological data. Clock analyses of multiple replicate transcriptome datasets suggest mid- to late-Pleistocene divergence dates within the H. tarsalis group complex. Coalescent and concatenated phylogenetic analyses indicate four early-diverging lineages (H. mustaciata, H. ophrys, and H. tarsalis from the Lahontan and Owens drainage basins), with remaining samples separated into larger clades from the Mojave desert, and western populations from the California Floristic Province of California and northern Baja California. -

Araneae, Salticidae)
Arachnologische Mitteilungen / Arachnology Letters 53: 62-66 Karlsruhe, April 2017 Ein Beitrag zur Springspinnenfauna Spaniens mit drei Erstnachweisen für die Balearen (Araneae, Salticidae) Michael Schäfer & Ernst Klimsa doi: 10.5431/aramit5310 Abstract. A contribution to the salticid fauna of Spain with three first records for the Balearic Islands (Araneae, Salticidae). In the course of several collection trips on the Spanish mainland and Mallorca, 18 species of jumping spiders were recorded, including three species discovered for the first time in the Balearic Islands: Hasarius adansoni (Audouin, 1826), Heliophanus ramosus Wesołowska, 1986 and Thyene imperialis (Rossi, 1846). In addition, the first European record of Heliophanus stylifer Simon, 1878 is corrected: it refers to a misidentified female of Heliophanus ramosus Wesołowska, 1986; accordingly, H. stylifer has to be removed from the European checklist. Keywords: distribution, Europe, faunistics, spider Zusammenfassung. Während mehrerer Exkursionen auf dem spanischen Festland und der Baleareninsel Mallorca wurden 18 Spring- spinnenarten nachgewiesen, darunter mit Hasarius adansoni (Audouin, 1826), Heliophanus ramosus Wesołowska, 1986 und Thyene im- perialis (Rossi, 1846) drei Arten erstmalig für die Balearen. Außerdem wird der europäische Erstnachweis von Heliophanus stylifer Simon, 1878 zu Heliophanus ramosus Wesołowska, 1986 korrigiert; H. stylifer muss dementsprechend von der europäischen Artenliste entfernt werden. Während mehrerer Exkursionen auf dem spanischen Fest- 2.363756°E, 350 m ü. NN, trockener Feldweg, unter Stein, ) land sowie der Baleareninsel Mallorca wurden zwischen 2013 26.12.2013: 1 ; Andalusien, bei San José, 36.800099°N und 2016 insgesamt 35 Springspinnen aus 18 Arten bzw. 14 2.068603°W, 20 m ü. NN, vegetationsarme Fläche, unter ) Gattungen gesammelt. Stein, 26.03.2016: 1 . -

70.1, 5 September 2008 ISSN 1944-8120
PECKHAMIA 70.1, 5 September 2008 ISSN 1944-8120 This is a PDF version of PECKHAMIA 3(2): 27-60, December 1995. Pagination of the original document has been retained. PECKHAMIA Volume 3 Number 2 Publication of the Peckham Society, an informal organization dedicated to research in the biology of jumping spiders. CONTENTS ARTICLES: A LIST OF THE JUMPING SPIDERS (SALTICIDAE) OF THE ISLANDS OF THE CARIBBEAN REGION G. B. Edwards and Robert J. Wolff..........................................................................27 DECEMBER 1995 A LIST OF THE JUMPING SPIDERS (SALTICIDAE) OF THE ISLANDS OF THE CARIBBEAN REGION G. B. Edwards Florida State Collection of Arthropods Division of Plant Industry P. O. Box 147100 Gainesville, FL 32614-7100 USA Robert J. Wolff1 Biology Department Trinity Christian College 6601 West College Drive Palos Heights, IL 60463 USA The following is a list of the jumping spiders that have been reported from the Caribbean region. We have interpreted this in a broad sense, so that all islands from Trinidad to the Bahamas have been included. Furthermore, we have included Bermuda, even though it is well north of the Caribbean region proper, as a more logical extension of the island fauna rather than the continental North American fauna. This was mentioned by Banks (1902b) nearly a century ago. Country or region (e. g., pantropical) records are included for those species which have broader ranges than the Caribbean area. We have not specifically included the islands of the Florida Keys, even though these could legitimately be included in the Caribbean region, because the known fauna is mostly continental. However, when Florida is known as the only continental U.S.A. -

A LIST of the JUMPING SPIDERS of MEXICO. David B. Richman and Bruce Cutler
PECKHAMIA 62.1, 11 October 2008 ISSN 1944-8120 This is a PDF version of PECKHAMIA 2(5): 63-88, December 1988. Pagination of the original document has been retained. 63 A LIST OF THE JUMPING SPIDERS OF MEXICO. David B. Richman and Bruce Cutler The salticids of Mexico are poorly known. Only a few works, such as F. O. Pickard-Cambridge (1901), have dealt with the fauna in any depth and these are considerably out of date. Hoffman (1976) included jumping spiders in her list of the spiders of Mexico, but the list does not contain many species known to occur in Mexico and has some synonyms listed. It is our hope to present a more complete list of Mexican salticids. Without a doubt such a work is preliminary and as more species are examined using modern methods a more complete picture of this varied fauna will emerge. The total of 200 species indicates more a lack of study than a sparse fauna. We would be surprised if the salticid fauna of Chiapas, for example, was not larger than for all of the United States. Unfortunately, much of the tropical forest may disappear before this fauna is fully known. The following list follows the general format of our earlier (1978) work on the salticid fauna of the United States and Canada. We have not prepared a key to genera, at least in part because of the obvious incompleteness of the list. We hope, however, that this list will stimulate further work on the Mexican salticid fauna. Acragas Simon 1900: 37. -

Checklist of the Spider Fauna of Bangladesh (Araneae : Arachnida)
Bangladesh J. Zool. 47(2): 185-227, 2019 ISSN: 0304-9027 (print) 2408-8455 (online) CHECKLIST OF THE SPIDER FAUNA OF BANGLADESH (ARANEAE : ARACHNIDA) Vivekanand Biswas* Department of Zoology, Khulna Government Womens’ College, Khulna-9000, Bangladesh Abstract: Spiders are one of the important predatory arthropods that comprise the largest order Araneae of the class Arachnida. In Bangladesh, very few contributions are available on the taxonomic study on these arachnids. The present paper contains an updated checklist of the spider fauna of Bangladesh based on the published records of different workers and the identified collections of the recent studies by the author. It includes a total of 334 species of spiders belong to the infraorders Mygalomorphae and Araneomorphae under 21 families and 100 genera. A brief diagnosis of different families and their domination together with the distribution throughout the country are provided herewith. Key words: Checklist, spiders, Araneae, Arachnida, Bangladesh INTRODUCTION Bangladesh is basically a riverine agricultural country. It lies between 20.35ºN and 26.75ºN latitude and 88.03ºE and 92.75ºE longitude, covering an area of 1,47,570 sq. km (55,126 sq. miles). The country as such offers varied climatic situations viz., temperature, rainfall, humidity, fogmist, dew and Haor- frost, winds etc. (Rashid 1977). With the vast agricultural lands, also there are different kinds of evergreen, deciduous and mangrove forests staying different areas of the country viz., the southern Sunderbans, northern Bhawal and Madhupur forests and eastern Chittagong and Chittagong Hill-Tracts forest. Along with the agricultural lands, each of the forest ecosystems is composed of numerous species of spider fauna of the country.