Chapter 41. One-Dimensional Quantum Mechanics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Accomplishments in Nanotechnology
U.S. Department of Commerce Carlos M. Gutierrez, Secretaiy Technology Administration Robert Cresanti, Under Secretaiy of Commerce for Technology National Institute ofStandards and Technolog}' William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment used are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publication 1052 Natl. Inst. Stand. Technol. Spec. Publ. 1052, 186 pages (August 2006) CODEN: NSPUE2 NIST Special Publication 1052 Accomplishments in Nanoteciinology Compiled and Edited by: Michael T. Postek, Assistant to the Director for Nanotechnology, Manufacturing Engineering Laboratory Joseph Kopanski, Program Office and David Wollman, Electronics and Electrical Engineering Laboratory U. S. Department of Commerce Technology Administration National Institute of Standards and Technology Gaithersburg, MD 20899 August 2006 National Institute of Standards and Teclinology • Technology Administration • U.S. Department of Commerce Acknowledgments Thanks go to the NIST technical staff for providing the information outlined on this report. Each of the investigators is identified with their contribution. Contact information can be obtained by going to: http ://www. nist.gov Acknowledged as well, -
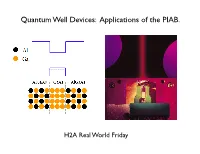
Quantum Well Devices: Applications of the PIAB
Quantum Well Devices: Applications of the PIAB. H2A Real World Friday What is a semiconductor? no e- Conduction Band Empty e levels a few e- Empty e levels lots of e- Empty e levels Filled e levels Filled e levels Filled e levels Insulator Semiconductor Metal Electrons in the conduction band of semiconductors like Si or GaAs can move about freely. Conduction band a few e- Eg = hν Energy Bandgap in Valence band Filled e levels Semiconductors We can get electrons into the conduction band by either thermal excitation or light excitation (photons). Solar cells use semiconductors to convert photons to electrons. A "quantum well" structure made from AlGaAs-GaAs-AlGaAs creates a potential well for conduction electrons. 10-20 nm! A conduction electron that get trapped in a quantum well acts like a PIAB. A conduction electron that get trapped in a quantum well acts like a PIAB. Quantum Wells are used to make Laser Diodes Quantum Well Laser Diodes Quantum Wells are used to make Laser Diodes Quantum Well Laser Diodes Multiple Quantum Wells work even better. Multiple Quantum Well Laser Diodes Multiple Quantum Wells work even better. Multiple Quantum Well LEDs Multiple Quantum Wells work even better. Multiple Quantum Well Laser Diodes Multiple Quantum Wells also are used to make high efficiency Solar Cells. Quantum Well Solar Cells Multiple Quantum Wells also are used to make high efficiency Solar Cells. The most common approach to high efficiency photovoltaic power conversion is to partition the solar spectrum into separate bands and each absorbed by a cell specially tailored for that spectral band. -

Federico Capasso “Physics by Design: Engineering Our Way out of the Thz Gap” Peter H
6 IEEE TRANSACTIONS ON TERAHERTZ SCIENCE AND TECHNOLOGY, VOL. 3, NO. 1, JANUARY 2013 Terahertz Pioneer: Federico Capasso “Physics by Design: Engineering Our Way Out of the THz Gap” Peter H. Siegel, Fellow, IEEE EDERICO CAPASSO1credits his father, an economist F and business man, for nourishing his early interest in science, and his mother for making sure he stuck it out, despite some tough moments. However, he confesses his real attraction to science came from a well read children’s book—Our Friend the Atom [1], which he received at the age of 7, and recalls fondly to this day. I read it myself, but it did not do me nearly as much good as it seems to have done for Federico! Capasso grew up in Rome, Italy, and appropriately studied Latin and Greek in his pre-university days. He recalls that his father wisely insisted that he and his sister become fluent in English at an early age, noting that this would be a more im- portant opportunity builder in later years. In the 1950s and early 1960s, Capasso remembers that for his family of friends at least, physics was the king of sciences in Italy. There was a strong push into nuclear energy, and Italy had a revered first son in En- rico Fermi. When Capasso enrolled at University of Rome in FREDERICO CAPASSO 1969, it was with the intent of becoming a nuclear physicist. The first two years were extremely difficult. University of exams, lack of grade inflation and rigorous course load, had Rome had very high standards—there were at least three faculty Capasso rethinking his career choice after two years. -

A Study of Fractional Schrödinger Equation-Composed Via Jumarie Fractional Derivative
A Study of Fractional Schrödinger Equation-composed via Jumarie fractional derivative Joydip Banerjee1, Uttam Ghosh2a , Susmita Sarkar2b and Shantanu Das3 Uttar Buincha Kajal Hari Primary school, Fulia, Nadia, West Bengal, India email- [email protected] 2Department of Applied Mathematics, University of Calcutta, Kolkata, India; 2aemail : [email protected] 2b email : [email protected] 3 Reactor Control Division BARC Mumbai India email : [email protected] Abstract One of the motivations for using fractional calculus in physical systems is due to fact that many times, in the space and time variables we are dealing which exhibit coarse-grained phenomena, meaning that infinitesimal quantities cannot be placed arbitrarily to zero-rather they are non-zero with a minimum length. Especially when we are dealing in microscopic to mesoscopic level of systems. Meaning if we denote x the point in space andt as point in time; then the differentials dx (and dt ) cannot be taken to limit zero, rather it has spread. A way to take this into account is to use infinitesimal quantities as ()Δx α (and ()Δt α ) with 01<α <, which for very-very small Δx (and Δt ); that is trending towards zero, these ‘fractional’ differentials are greater that Δx (and Δt ). That is()Δx α >Δx . This way defining the differentials-or rather fractional differentials makes us to use fractional derivatives in the study of dynamic systems. In fractional calculus the fractional order trigonometric functions play important role. The Mittag-Leffler function which plays important role in the field of fractional calculus; and the fractional order trigonometric functions are defined using this Mittag-Leffler function. -

Lecture 16 Matter Waves & Wave Functions
LECTURE 16 MATTER WAVES & WAVE FUNCTIONS Instructor: Kazumi Tolich Lecture 16 2 ¨ Reading chapter 34-5 to 34-8 & 34-10 ¤ Electrons and matter waves n The de Broglie hypothesis n Electron interference and diffraction ¤ Wave-particle duality ¤ Standing waves and energy quantization ¤ Wave function ¤ Uncertainty principle ¤ A particle in a box n Standing wave functions de Broglie hypothesis 3 ¨ In 1924 Louis de Broglie hypothesized: ¤ Since light exhibits particle-like properties and act as a photon, particles could exhibit wave-like properties and have a definite wavelength. ¨ The wavelength and frequency of matter: # & � = , and � = $ # ¤ For macroscopic objects, de Broglie wavelength is too small to be observed. Example 1 4 ¨ One of the smallest composite microscopic particles we could imagine using in an experiment would be a particle of smoke or soot. These are about 1 µm in diameter, barely at the resolution limit of most microscopes. A particle of this size with the density of carbon has a mass of about 10-18 kg. What is the de Broglie wavelength for such a particle, if it is moving slowly at 1 mm/s? Diffraction of matter 5 ¨ In 1927, C. J. Davisson and L. H. Germer first observed the diffraction of electron waves using electrons scattered from a particular nickel crystal. ¨ G. P. Thomson (son of J. J. Thomson who discovered electrons) showed electron diffraction when the electrons pass through a thin metal foils. ¨ Diffraction has been seen for neutrons, hydrogen atoms, alpha particles, and complicated molecules. ¨ In all cases, the measured λ matched de Broglie’s prediction. X-ray diffraction electron diffraction neutron diffraction Interference of matter 6 ¨ If the wavelengths are made long enough (by using very slow moving particles), interference patters of particles can be observed. -

Dephasing in an Electronic Mach-Zehnder Interferometer,” Phys
Dephasing and Quantum Noise in an electronic Mach-Zehnder Interferometer Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) der Fakultät für Physik der Universität Regensburg vorgelegt von Andreas Helzel aus Kelheim Dezember 2012 ii Promotionsgesuch eingereicht am: 22.11.2012 Die Arbeit wurde angeleitet von: Prof. Dr. Christoph Strunk Prüfungsausschuss: Prof. Dr. G. Bali (Vorsitzender) Prof. Dr. Ch. Strunk (1. Gutachter) Dr. F. Pierre (2. Gutachter) Prof. Dr. F. Gießibl (weiterer Prüfer) iii In Gedenken an Maria Höpfl. Sie war die erste Taxifahrerin von Kelheim und wurde 100 Jahre alt. Contents 1. Introduction1 2. Basics 5 2.1. The two dimensional electron gas....................5 2.2. The quantum Hall effect - Quantized Landau levels...........8 2.3. Transport in the quantum Hall regime.................. 10 2.3.1. Quantum Hall edge states and Landauer-Büttiker formalism.. 10 2.3.2. Compressible and incompressible strips............. 14 2.3.3. Luttinger liquid in the QH regime at filling factor 2....... 16 2.4. Non-equilibrium fluctuations of a QPC.................. 24 2.5. Aharonov-Bohm Interferometry..................... 28 2.6. The electronic Mach-Zehnder interferometer............... 30 3. Measurement techniques 37 3.1. Cryostat and devices........................... 37 3.2. Measurement approach.......................... 39 4. Sample fabrication and characterization 43 4.1. Fabrication................................ 43 4.1.1. Material.............................. 43 4.1.2. Lithography............................ 44 4.1.3. Gold air bridges.......................... 45 4.1.4. Sample Design.......................... 46 4.2. Characterization.............................. 47 4.2.1. Filling factor........................... 47 4.2.2. Quantum point contacts..................... 49 4.2.3. Gate setting............................ 50 5. Characteristics of a MZI 51 5.1. -

Bohr's Correspondence Principle
Bohr’s Correspondence Principle Bohr's theory was deliberately incomplete so that he could find principles that would allow a systematic search for a “rational generalization” of classical electrodynamics. A major conceptual tool that characterized Bohr’s atomic work was the correspondence principle, his celebrated asymptotic consistency requirement between quantum theory and classical physics, that took various forms over the years and was often misunderstood by even his closest collaborators [312, p. 114], but guided his research and dominated quantum theory until the emergence of quantum mechanics in 1925-26 [419, p. 81]. It should be emphasized that the correspondence principle is not the elementary recognition that the results of classical and quantum physics converge in the limit ℎ → 0. Bohr said emphatically that this [420] See the print edition of The Quantum Measurement Problem for quotation. Instead, the correspondence principle is deeper and aims at the heart of the differences between quantum and classical physics. Even more than the previous fundamental work of Planck and Einstein on the quantum, Bohr’s atomic work marked a decisive break with classical physics. This was primarily because from the second postulate of Bohr’s theory there can be no relation between the light frequency (or color of the light) and the period of the electron, and thus the theory differed in principle from the classical picture. This disturbed many physicists at the time since experiments had confirmed the classical relation between the period of the waves and the electric currents. However, Bohr was able to show that if one considers increasingly larger orbits characterized by quantum number n, two neighboring orbits will have periods that approach a common value. -

Higher Order Schrödinger Equations Rémi Carles, Emmanuel Moulay
Higher order Schrödinger equations Rémi Carles, Emmanuel Moulay To cite this version: Rémi Carles, Emmanuel Moulay. Higher order Schrödinger equations. Journal of Physics A: Mathematical and Theoretical, IOP Publishing, 2012, 45 (39), pp.395304. 10.1088/1751- 8113/45/39/395304. hal-00776157 HAL Id: hal-00776157 https://hal.archives-ouvertes.fr/hal-00776157 Submitted on 12 Feb 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. HIGHER ORDER SCHRODINGER¨ EQUATIONS REMI´ CARLES AND EMMANUEL MOULAY Abstract. The purpose of this paper is to provide higher order Schr¨odinger equations from a finite expansion approach. These equations converge toward the semi-relativistic equation for particles whose norm of their velocity vector c is below √2 and are able to take into account some relativistic effects with a certain accuracy in a sense that we define. So, it is possible to take into account some relativistic effects by using Schr¨odinger form equations, even if they cannot be considered as relativistic wave equations. 1. Introduction In quantum mechanics, the Schr¨odinger equation is a fundamental non-relativistic quantum equation that describes how the wave-function of a physical system evolves over time. -

Dirac Particle in a Square Well and in a Box
Dirac particle in a square well and in a box A. D. Alhaidari Saudi Center for Theoretical Physics, Dhahran, Saudi Arabia We obtain an exact solution of the 1D Dirac equation for a square well potential of depth greater then twice the particle’s mass. The energy spectrum formula in the Klein zone is surprisingly very simple and independent of the depth of the well. This implies that the same solution is also valid for the potential box (infinitely deep well). In the nonrelativistic limit, the well-known energy spectrum of a particle in a box is obtained. We also provide in tabular form the elements of the complete solution space of the problem for all energies. PACS numbers: 03.65.Pm, 03.65.Ge, 03.65.Nk Keywords: Dirac equation, square well, potential box, energy spectrum, Klein paradox Aside from the mathematically “trivial” free case, particle in a box is usually the first problem that an undergraduate student of nonrelativistic quantum mechanics is asked to solve. Normally, he goes further into obtaining the bound states solution of a particle in a finite square well. It is much later that he works out more involved exercises like the 3D Coulomb problem [1]. Unfortunately, in relativistic quantum mechanics the story is reversed. One can hardly find a textbook on relativistic quantum mechanics where the 1D problem of a particle in a potential box is solved before the relativistic Hydrogen atom is [2]. The difficulty is that if this is to be done from the start, then one is forced to get into subtle issues like the Klein paradox, electron-positron pair production, stability of the vacuum, appropriate boundary conditions, …etc. -

Quantum Mechanics
Quantum Mechanics Richard Fitzpatrick Professor of Physics The University of Texas at Austin Contents 1 Introduction 5 1.1 Intendedaudience................................ 5 1.2 MajorSources .................................. 5 1.3 AimofCourse .................................. 6 1.4 OutlineofCourse ................................ 6 2 Probability Theory 7 2.1 Introduction ................................... 7 2.2 WhatisProbability?.............................. 7 2.3 CombiningProbabilities. ... 7 2.4 Mean,Variance,andStandardDeviation . ..... 9 2.5 ContinuousProbabilityDistributions. ........ 11 3 Wave-Particle Duality 13 3.1 Introduction ................................... 13 3.2 Wavefunctions.................................. 13 3.3 PlaneWaves ................................... 14 3.4 RepresentationofWavesviaComplexFunctions . ....... 15 3.5 ClassicalLightWaves ............................. 18 3.6 PhotoelectricEffect ............................. 19 3.7 QuantumTheoryofLight. .. .. .. .. .. .. .. .. .. .. .. .. .. 21 3.8 ClassicalInterferenceofLightWaves . ...... 21 3.9 QuantumInterferenceofLight . 22 3.10 ClassicalParticles . .. .. .. .. .. .. .. .. .. .. .. .. .. .. 25 3.11 QuantumParticles............................... 25 3.12 WavePackets .................................. 26 2 QUANTUM MECHANICS 3.13 EvolutionofWavePackets . 29 3.14 Heisenberg’sUncertaintyPrinciple . ........ 32 3.15 Schr¨odinger’sEquation . 35 3.16 CollapseoftheWaveFunction . 36 4 Fundamentals of Quantum Mechanics 39 4.1 Introduction .................................. -

Electric Polarizability in the Three Dimensional Problem and the Solution of an Inhomogeneous Differential Equation
Electric polarizability in the three dimensional problem and the solution of an inhomogeneous differential equation M. A. Maizea) and J. J. Smetankab) Department of Physics, Saint Vincent College, Latrobe, PA 15650 USA In previous publications, we illustrated the effectiveness of the method of the inhomogeneous differential equation in calculating the electric polarizability in the one-dimensional problem. In this paper, we extend our effort to apply the method to the three-dimensional problem. We calculate the energy shift of a quantum level using second-order perturbation theory. The energy shift is then used to calculate the electric polarizability due to the interaction between a static electric field and a charged particle moving under the influence of a spherical delta potential. No explicit use of the continuum states is necessary to derive our results. I. INTRODUCTION In previous work1-3, we employed the simple and elegant method of the inhomogeneous differential equation4 to calculate the energy shift of a quantum level in second-order perturbation theory. The energy shift was a result of an interaction between an applied static electric field and a charged particle moving under the influence of a one-dimensional bound 1 potential. The electric polarizability, , was obtained using the basic relationship ∆퐸 = 휖2 0 2 with ∆퐸0 being the energy shift and is the magnitude of the applied electric field. The method of the inhomogeneous differential equation devised by Dalgarno and Lewis4 and discussed by Schwartz5 can be used in a large variety of problems as a clever replacement for conventional perturbation methods. As we learn in our introductory courses in quantum mechanics, calculating the energy shift in second-order perturbation using conventional methods involves a sum (which can be infinite) or an integral that contains all possible states allowed by the transition. -

Revisiting Double Dirac Delta Potential
Revisiting double Dirac delta potential Zafar Ahmed1, Sachin Kumar2, Mayank Sharma3, Vibhu Sharma3;4 1Nuclear Physics Division, Bhabha Atomic Research Centre, Mumbai 400085, India 2Theoretical Physics Division, Bhabha Atomic Research Centre, Mumbai 400085, India 3;4Amity Institute of Applied Sciences, Amity University, Noida, UP, 201313, India∗ (Dated: June 14, 2016) Abstract We study a general double Dirac delta potential to show that this is the simplest yet versatile solvable potential to introduce double wells, avoided crossings, resonances and perfect transmission (T = 1). Perfect transmission energies turn out to be the critical property of symmetric and anti- symmetric cases wherein these discrete energies are found to correspond to the eigenvalues of Dirac delta potential placed symmetrically between two rigid walls. For well(s) or barrier(s), perfect transmission [or zero reflectivity, R(E)] at energy E = 0 is non-intuitive. However, earlier this has been found and called \threshold anomaly". Here we show that it is a critical phenomenon and we can have 0 ≤ R(0) < 1 when the parameters of the double delta potential satisfy an interesting condition. We also invoke zero-energy and zero curvature eigenstate ( (x) = Ax + B) of delta well between two symmetric rigid walls for R(0) = 0. We resolve that the resonant energies and the perfect transmission energies are different and they arise differently. arXiv:1603.07726v4 [quant-ph] 13 Jun 2016 ∗Electronic address: 1:[email protected], 2: [email protected], 3: [email protected], 4:[email protected] 1 I. INTRODUCTION The general one-dimensional Double Dirac Delta Potential (DDDP) is written as [see Fig.