Germination Responses of a Dry Sclerophyll Forest Soil-Stored Seedbank to Fire Related Cues
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blue Tier Reserve Background Report 2016File
Background Report Blue Tier Reserve www.tasland.org.au Tasmanian Land Conservancy (2016). The Blue Tier Reserve Background Report. Tasmanian Land Conservancy, Tasmania Australia. Copyright ©Tasmanian Land Conservancy The views expressed in this report are those of the Tasmanian Land Conservancy and not the Federal Government, State Government or any other entity. This work is copyright. It may be reproduced for study, research or training purposes subject to an acknowledgment of the sources and no commercial usage or sale. Requests and enquires concerning reproduction and rights should be addressed to the Tasmanian Land Conservancy. Front Image: Myrtle rainforest on Blue Tier Reserve - Andy Townsend Contact Address Tasmanian Land Conservancy PO Box 2112, Lower Sandy Bay, 827 Sandy Bay Road, Sandy Bay TAS 7005 | p: 03 6225 1399 | www.tasland.org.au Contents Acknowledgements ................................................................................................................................. 1 Acronyms and Abbreviations .......................................................................................................... 2 Introduction ............................................................................................................................................ 3 Location and Access ................................................................................................................................ 4 Bioregional Values and Reserve Status .................................................................................................. -

Winter Edition 2020 - 3 in This Issue: Office Bearers for 2017
1 Australian Plants Society Armidale & District Group PO Box 735 Armidale NSW 2350 web: www.austplants.com.au/Armidale e-mail: [email protected] Crowea exalata ssp magnifolia image by Maria Hitchcock Winter Edition 2020 - 3 In this issue: Office bearers for 2017 ......p1 Editorial …...p2Error! Bookmark not defined. New Website Arrangements .…..p3 Solstice Gathering ......p4 Passion, Boers & Hibiscus ......p5 Wollomombi Falls Lookout ......p7 Hard Yakka ......p8 Torrington & Gibraltar after fires ......p9 Small Eucalypts ......p12 Drought tolerance of plants ......p15 Armidale & District Group PO Box 735, Armidale NSW 2350 President: Vacant Vice President: Colin Wilson Secretary: Penelope Sinclair Ph. 6771 5639 [email protected] Treasurer: Phil Rose Ph. 6775 3767 [email protected] Membership: Phil Rose [email protected] 2 Markets in the Mall, Outings, OHS & Environmental Officer and Arboretum Coordinator: Patrick Laher Ph: 0427327719 [email protected] Newsletter Editor: John Nevin Ph: 6775218 [email protected],net.au Meet and Greet: Lee Horsley Ph: 0421381157 [email protected] Afternoon tea: Deidre Waters Ph: 67753754 [email protected] Web Master: Eric Sinclair Our website: http://www.austplants.com.au From the Editor: We have certainly had a memorable year - the worst drought in living memory followed by the most extensive bushfires seen in Australia, and to top it off, the biggest pandemic the world has seen in 100 years. The pandemic has made essential self distancing and quarantining to arrest the spread of the Corona virus. As a result, most APS activities have been shelved for the time being. Being in isolation at home has been a mixed blessing. -

(Hymenoptera: Eurytomidae) in the Integrated Control of Acacia Species in South Africa
Proceedings of the X International Symposium on Biological Control of Weeds 919 4-14 July 1999, Montana State University, Bozeman, Montana, USA Neal R. Spencer [ed.]. pp. 919-929 (2000) The Potential Role of Bruchophagus acaciae (Cameron) (Hymenoptera: Eurytomidae) in the Integrated Control of Acacia Species in South Africa R. L. HILL1, A. J. GORDON2, and S. NESER3 1Richard Hill & Associates, Private Bag 4704, Christchurch, New Zealand 2Plant Protection Research Institute, Private Bag X5017, Stellenbosch, 7599 South Africa 3Plant Protection Research Institute, Private Bag X134, Pretoria, 0001 South Africa Abstract Australian acacias invade watersheds and riverbeds in South Africa, reducing water flows and threatening environmental and economic values. Acacia mearnsii is the most widespread and important weed but also forms the basis of an important industry. A. dealbata, and to a lesser extent A. decurrens are also problems. All belong to the Section Botrycephalae of the sub-genus Heterophyllum. Short term control is achieved locally by removing plants, and by using herbicides, but seed-feeding control agents may provide an acceptable solution in the long term. Larvae of Bruchophagus acaciae (Cameron) (Hymenoptera: Eurytomidae) develop in the seeds of acacias. It was described from New Zealand, but is an Australian species. We explore whether B. acaciae has a role as a con- trol agent for acacias in South Africa. Seed was collected from 28 Australian species of Acacia growing in New Zealand. Attack was restricted to four of the seven species with- in the Section Botrycephalae, and two cases of attack on Acacia rubida (Section Phyllodineae; n=9). Apart from a wasp reared from one seed, A. -

Wattles of the City of Whittlesea
Wattles of the City of Whittlesea PROTECTING BIODIVERSITY ON PRIVATE LAND SERIES Wattles of the City of Whittlesea Over a dozen species of wattle are indigenous to the City of Whittlesea and many other wattle species are commonly grown in gardens. Most of the indigenous species are commonly found in the forested hills and the native forests in the northern parts of the municipality, with some species persisting along country roadsides, in smaller reserves and along creeks. Wattles are truly amazing • Wattles have multiple uses for Australian plants indigenous peoples, with most species used for food, medicine • There are more wattle species than and/or tools. any other plant genus in Australia • Wattle seeds have very hard coats (over 1000 species and subspecies). which mean they can survive in the • Wattles, like peas, fix nitrogen in ground for decades, waiting for a the soil, making them excellent cool fire to stimulate germination. for developing gardens and in • Australia’s floral emblem is a wattle: revegetation projects. Golden Wattle (Acacia pycnantha) • Many species of insects (including and this is one of Whittlesea’s local some butterflies) breed only on species specific species of wattles, making • In Victoria there is at least one them a central focus of biodiversity. wattle species in flower at all times • Wattle seeds and the insects of the year. In the Whittlesea attracted to wattle flowers are an area, there is an indigenous wattle important food source for most bird in flower from February to early species including Black Cockatoos December. and honeyeaters. Caterpillars of the Imperial Blue Butterfly are only found on wattles RB 3 Basic terminology • ‘Wattle’ = Acacia Wattle is the common name and Acacia the scientific name for this well-known group of similar / related species. -

The Native Vegetation of the Nattai and Bargo Reserves
The Native Vegetation of the Nattai and Bargo Reserves Project funded under the Central Directorate Parks and Wildlife Division Biodiversity Data Priorities Program Conservation Assessment and Data Unit Conservation Programs and Planning Branch, Metropolitan Environmental Protection and Regulation Division Department of Environment and Conservation ACKNOWLEDGMENTS CADU (Central) Manager Special thanks to: Julie Ravallion Nattai NP Area staff for providing general assistance as well as their knowledge of the CADU (Central) Bioregional Data Group area, especially: Raf Pedroza and Adrian Coordinator Johnstone. Daniel Connolly Citation CADU (Central) Flora Project Officer DEC (2004) The Native Vegetation of the Nattai Nathan Kearnes and Bargo Reserves. Unpublished Report. Department of Environment and Conservation, CADU (Central) GIS, Data Management and Hurstville. Database Coordinator This report was funded by the Central Peter Ewin Directorate Parks and Wildlife Division, Biodiversity Survey Priorities Program. Logistics and Survey Planning All photographs are held by DEC. To obtain a Nathan Kearnes copy please contact the Bioregional Data Group Coordinator, DEC Hurstville Field Surveyors David Thomas Cover Photos Teresa James Nathan Kearnes Feature Photo (Daniel Connolly) Daniel Connolly White-striped Freetail-bat (Michael Todd), Rock Peter Ewin Plate-Heath Mallee (DEC) Black Crevice-skink (David O’Connor) Aerial Photo Interpretation Tall Moist Blue Gum Forest (DEC) Ian Roberts (Nattai and Bargo, this report; Rainforest (DEC) Woronora, 2003; Western Sydney, 1999) Short-beaked Echidna (D. O’Connor) Bob Wilson (Warragamba, 2003) Grey Gum (Daniel Connolly) Pintech (Pty Ltd) Red-crowned Toadlet (Dave Hunter) Data Analysis ISBN 07313 6851 7 Nathan Kearnes Daniel Connolly Report Writing and Map Production Nathan Kearnes Daniel Connolly EXECUTIVE SUMMARY This report describes the distribution and composition of the native vegetation within and immediately surrounding Nattai National Park, Nattai State Conservation Area and Bargo State Conservation Area. -

Muelleria : an Australian Journal of Botany
Muelleria Volume 5 Number 1 March, 1982 NATIONAL HERBARIUM OF VICTORIA DEPARTMENT OF CROWN LANDS AND SURVEY Muelleria Volume 5, Number 1 March, 1982 CONTENTS Page A revision of the genus Templelonia R.Br. (Papilionaceae) — J. H. Ross 1 The nomenclature of some Australian lichens described as Lecanora and Placodium by Miiller-Argoviensis — R. W. Rogers 31 New Australian species of Nymphoides Seguier (Menyanthaceae) — Helen 1. Aston 35 Vegetation of East Gippsland — S. J. Forbes, N. G. Walsh and P. K. Gullan 53 A new Australian lichen: Cladonia sulcata — A. W. Archer 115 Editor: Helen 1. Aston Published by the National Herbarium of Victoria (MEL). Royal Botanic Gardens, South Yarra, Victoria 3141, Australia. D. M. Churchill, Director and Government Botanist. 43346/81 The date of publication of Volume 4, number 4, was 20 May 1981. A REVISION OF THE GENUS TEMPLETONIA R.Br. (PAPILIONACEAE) by J. H. Ross* ABSTRACT The endemic Australian genus Templetonia is revised. Eleven species are recognized and the uncertainty concerning the application of the name T. sulcata (Meissn.) Benth. is discussed. This discussion includes the selection ol a lectotype for Bossiaea rossii F. Muell., a possible synonym. Descriptions, a key to the identification of species, illustrations, and distribution maps are provided, together with notes on ecology and relationships. Two previous papers describing T. incana (.Muelleria 4: 247-249 (1980)) and T. negketa (loc. cit. 390-393 (1981)) should be used in conjunction with the present revision. INTRODUCTION Templetonia, a small genus of 1 1 species described by R. Brown in Ait. f Hort. , Kew. ed. 2, 4: 269 (1812), was named in honour of the Irish botanist John Templeton (1776-1825) ot Orange Grove, Belfast. -
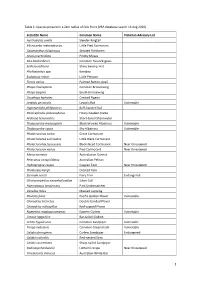
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Indigenous Plants of Bendigo
Produced by Indigenous Plants of Bendigo Indigenous Plants of Bendigo PMS 1807 RED PMS 432 GREY PMS 142 GOLD A Gardener’s Guide to Growing and Protecting Local Plants 3rd Edition 9 © Copyright City of Greater Bendigo and Bendigo Native Plant Group Inc. This work is Copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the City of Greater Bendigo. First Published 2004 Second Edition 2007 Third Edition 2013 Printed by Bendigo Modern Press: www.bmp.com.au This book is also available on the City of Greater Bendigo website: www.bendigo.vic.gov.au Printed on 100% recycled paper. Disclaimer “The information contained in this publication is of a general nature only. This publication is not intended to provide a definitive analysis, or discussion, on each issue canvassed. While the Committee/Council believes the information contained herein is correct, it does not accept any liability whatsoever/howsoever arising from reliance on this publication. Therefore, readers should make their own enquiries, and conduct their own investigations, concerning every issue canvassed herein.” Front cover - Clockwise from centre top: Bendigo Wax-flower (Pam Sheean), Hoary Sunray (Marilyn Sprague), Red Ironbark (Pam Sheean), Green Mallee (Anthony Sheean), Whirrakee Wattle (Anthony Sheean). Table of contents Acknowledgements ...............................................2 Foreword..........................................................3 Introduction.......................................................4 -

Appendix 3 Section 5A Assessments “Seven Part Tests”
APPENDIX 3 SECTION 5A ASSESSMENTS “SEVEN PART TESTS” Appendix 3: Seven Part Tests Swamp Sclerophyll Forest Swamp Sclerophyll Forest on Coastal Floodplains of the NSW North Coast, Sydney Basin and South East Corner bioregions is listed as an Endangered Ecological Community under the NSW Threatened Species Conservation Act (1995). It is not listed under the schedules of the Commonwealth Environmental Protection and Biodiversity Conservation Act (1999). Swamp Sclerophyll Forest on Coastal Floodplains of the NSW North Coast, Sydney Basin and South East Corner bioregions includes and replaces Sydney Coastal Estuary Swamp Forest in the Sydney Basin bioregion Endangered Ecological Community. This community is associated with humic clay loams and sandy loams, on waterlogged or periodically inundated alluvial flats and drainage lines associated with coastal floodplains (NSW Scientific Committee 2011). It occurs typically as open forests to woodlands, although partial clearing may have reduced the canopy to scattered trees or scrub. The understorey may contain areas of fernland and tall reedland or sedgeland which in turn may also form mosaics with other floodplain communities and often fringe wetlands with semi-permanent standing water (NSW Scientific Committee 2011). Swamp Sclerophyll Forest on Coastal Floodplains generally occurs below 20 metres ASL, often on small floodplains or where the larger floodplains adjoin lithic substrates or coastal sand plains (NSW Scientific Committee 2011). The species composition of Swamp Sclerophyll Forest is primarily determined by the frequency and duration of waterlogging and the texture, salinity nutrient and moisture content of the soil. The species composition of the trees varies considerably, but the most widespread and abundant dominant trees include Eucalyptus robusta Swamp Mahogany, Melaleuca quinquenervia and, south from Sydney, Eucalyptus botryoides Bangalay and Eucalyptus longifolia Woollybutt (OEH 2015a). -

Phytophthora Resistance and Susceptibility Stock List
Currently known status of the following plants to Phytophthora species - pathogenic water moulds from the Agricultural Pathology & Kingdom Protista. Biological Farming Service C ompiled by Dr Mary Cole, Agpath P/L. Agricultural Consultants since 1980 S=susceptible; MS=moderately susceptible; T= tolerant; MT=moderately tolerant; ?=no information available. Phytophthora status Life Form Botanical Name Family Common Name Susceptible (S) Tolerant (T) Unknown (UnK) Shrub Acacia brownii Mimosaceae Heath Wattle MS Tree Acacia dealbata Mimosaceae Silver Wattle T Shrub Acacia genistifolia Mimosaceae Spreading Wattle MS Tree Acacia implexa Mimosaceae Lightwood MT Tree Acacia leprosa Mimosaceae Cinnamon Wattle ? Tree Acacia mearnsii Mimosaceae Black Wattle MS Tree Acacia melanoxylon Mimosaceae Blackwood MT Tree Acacia mucronata Mimosaceae Narrow Leaf Wattle S Tree Acacia myrtifolia Mimosaceae Myrtle Wattle S Shrub Acacia myrtifolia Mimosaceae Myrtle Wattle S Tree Acacia obliquinervia Mimosaceae Mountain Hickory Wattle ? Shrub Acacia oxycedrus Mimosaceae Spike Wattle S Shrub Acacia paradoxa Mimosaceae Hedge Wattle MT Tree Acacia pycnantha Mimosaceae Golden Wattle S Shrub Acacia sophorae Mimosaceae Coast Wattle S Shrub Acacia stricta Mimosaceae Hop Wattle ? Shrubs Acacia suaveolens Mimosaceae Sweet Wattle S Tree Acacia ulicifolia Mimosaceae Juniper Wattle S Shrub Acacia verniciflua Mimosaceae Varnish wattle S Shrub Acacia verticillata Mimosaceae Prickly Moses ? Groundcover Acaena novae-zelandiae Rosaceae Bidgee-Widgee T Tree Allocasuarina littoralis Casuarinaceae Black Sheoke S Tree Allocasuarina paludosa Casuarinaceae Swamp Sheoke S Tree Allocasuarina verticillata Casuarinaceae Drooping Sheoak S Sedge Amperea xipchoclada Euphorbaceae Broom Spurge S Grass Amphibromus neesii Poaceae Swamp Wallaby Grass ? Shrub Aotus ericoides Papillionaceae Common Aotus S Groundcover Apium prostratum Apiaceae Sea Celery MS Herb Arthropodium milleflorum Asparagaceae Pale Vanilla Lily S? Herb Arthropodium strictum Asparagaceae Chocolate Lily S? Shrub Atriplex paludosa ssp. -

Structural and Floristic Variation in the Forest Communities of the West Tamar, Tasmania
Papers and Proceedings of the Royal Society of Tasmania, Volume 117, 1983. [ms. received 3.9.1982) STRUCTURAL AND FLORISTIC VARIATION IN THE FOREST COMMUNITIES OF THE WEST TAMAR, TASMANIA. by M.J. Brown and R.T. Buckney N.P.W.S., P.O. Box 210, Sandy Bay, Tas. 7005 and School of Life Sciences, N.S.W. Inst. Tech., P.O. Box 123, Broadway, N.S.W. 2007. (with three tables and five text-figures) ABSTRACT BROWN, M.J. & BUCKNEY, R.T., 1983 (31 viii): Structural and floristic variation in the forest communities of the West Tamar, Tasmania. Pap. Proc. R. Soc. Tasm., 117: 135- 152. https://doi.org/10.26749/rstpp.117.135 ISSN 0080-4703. National Parks & Wildlife Service, P.O. Box 210, Sandy Bay, Tasmania and School of Life Sciences, N.S.W. Institute of Technology, P.O. Box 123, Broadway, New South Wales. The forest communities in the West Tamar were sampled by a stratified random process using 55 plots selected after preliminary analy sis of 243 Forestry Commission continuous forest inventory plots, which occur in the area. A total of 13 floristic units were recognised and described. The relationship of the floristic units to changing water availability, drainage, soil fertility and fire frequency are assessed and the problems of structural versus floristic classifications of forest types are discussed. INTRODUCTION The dry forests of Tasmania occupy some of the most heavily utilised areas of the state. Clearing for agriculture and urban development, sawlog extraction, firewood getting and burning combined with stock grazing have all made their mark on this vegeta tion.