Safe Disposal of Unused Controlled Substances/ Current Challenges and Opportunities for Reform
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MEDICINE DISPOSAL PRODUCTS an Overview of Products & Performance Questions
MEDICINE DISPOSAL PRODUCTS an overview of products & performance questions Community Environmental Health Strategies LLC March Produced for San Francisco Department of the Environment 2019 Table of Contents Executive Summary .............................................................................................................................................. 2 Glossary of Abbreviations ..................................................................................................................................................... 4 I. Overview of Products and Questions Examined ..................................................................................... 5 II. Medicine Disposal Background ................................................................................................................... 8 A. Medicine Disposal Guidelines and Regulations............................................................................................... 8 B. DEA’s non-retrievable standard for controlled substances .................................................................... 10 C. Disposal of waste pharmaceuticals from households................................................................................ 11 D. Disposal of waste pharmaceuticals by the healthcare sector and regulated generators ........... 17 III. Medicine Disposal Products: Performance Questions & Available Product Testing ............ 23 A. Performance Questions ......................................................................................................................................... -

Legal Issues Relating to the Disposal of Dispensed Controlled Substances
. Legal Issues Relating to the Disposal of Dispensed Controlled Substances Brian T. Yeh Legislative Attorney July 30, 2010 Congressional Research Service 7-5700 www.crs.gov R40548 CRS Report for Congress Prepared for Members and Committees of Congress c11173008 . Legal Issues Relating to the Disposal of Dispensed Controlled Substances Summary According to the White House Office of National Drug Control Policy, the intentional use of prescription drugs for non-medical purposes is the fastest-growing drug problem in the country and the second-most common form of illicit drug abuse among teenagers in the United States, behind marijuana use. Young adults and teenagers may find their parents’ prescription drugs in unsecured medicine cabinets or other obvious locations in the home, or they may retrieve expired or unwanted medication from the trash. It is believed that properly disposing of unwanted medications would help prevent prescription drug abuse by reducing the accessibility and availability of such drugs. Yet throwing prescription medications into the trash or flushing them down the toilet may not be environmentally responsible. In response, many local communities and states have implemented pharmaceutical disposal programs (often referred to as drug “take- back” programs) that collect unused and unwanted medications from patients for incineration or other method of destruction that complies with federal and state laws and regulations, including those relating to public health and the environment. Prescription drugs may be categorized as either controlled substance medication or non- controlled substance medication. Pharmaceutical controlled substances, such as narcotic pain relievers OxyContin® and Vicodin®, are among the most commonly abused prescription drugs. -

Emergence of an Information Infrastructure Through Integrating Waste Drug Recycling, Medication Management, and Household Drug Management in China
Proceedings of the 52nd Hawaii International Conference on System Sciences | 2019 Emergence of an Information Infrastructure through Integrating Waste Drug Recycling, Medication Management, and Household Drug Management in China Yumei Luo Kai Reimers College of Business and Tourism Management, School of Business and Economics Yunnan University RWTH Aachen University [email protected] [email protected] Abstract In China, storing drugs for emergent use in homes in large quantities and varieties has become a common The total amount of waste drugs is expanding practice. With the rapid economic and social significantly as populations age and societies become development of society the total amount of household wealthier. Drug waste is becoming a problem for waste drugs is expanding significantly. In 2014, a health and environment. Thus, how to reduce and survey showed that about 78.6% of families in China effectively dispose of waste drugs is increasingly have the habit of storing prescription medicine and becoming an issue for society. In this paper, we more than 80% of the families do not regularly purge analyze the current situation with regard to existing their stock from expired medicines and even fewer systems for expired drug recycling and disposal in people know how to properly dispose of the expired China and suggest that by connecting the involved medicine [7]. The survey also showed that 72.5% of practices of waste drug recycling, medication families throw expired drugs into regular household management, and household drug management the waste, 5% flush them down the toilet or drain, and 6% incentives to participate in an integrated drug of families even continue to use them. -

Safe Disposal of Unused Controlled Substances
Safe Disposal of Unused Controlled Substances/ Current Challenges and Opportunities for Reform Prepared for: King Pharmaceuticals, Inc. Prepared by: Sharon Siler Suzanne Duda Ruth Brown Josette Gbemudu Scott Weier Jon Glaudemans King Pharmaceuticals, Inc. provided funding for this research. Avalere Health maintained full editorial control and the conclusions expressed here are those of the authors. TABLE OF CONTENTS 2 EXECUTIVE SUMMARY 6 INTRODUCTION 14 METHODOLOGY 18 CURRENT LANDSCAPE FOR DRUG DISPOSAL PROGRAMS 32 CRITICAL SUCCESS FACTORS 52 MODELS FOR REFORM 60 CONCLUSION 62 ACKNOWLEDGEMENTS 64 ENDNOTES King Pharmaceuticals, Inc. provided funding for this research. Avalere Health maintained full editorial control and the conclusions expressed here are those of the authors. EXECUTIVE SUMMARY SAFE DISPOSAL OF UNUSED CONTROLLED SUBSTANCES Disposal of unused prescription drugs, and controlled substances in particular, is a complicated issue. Unused drug take-back programs are emerging across the country as one strategy for reducing drug abuse, accidental poison- ing, and flushing drugs into the water supply. Current laws and regulations regarding controlled substances, however, limit these programs from accepting all drugs without strict oversight from law enforcement. SIGNIFICANT BARRIERS The Controlled Substances Act and Drug Enforcement Agency regulations dictate who can handle controlled substances, and are two of the most significant challenges today facing efforts to dispose of unused drugs. The law and regulations prohibit pharmacies, providers, and hospitals from collecting controlled substances that have already been dispensed to consumers. There is an exception that allows law enforcement officers to accept controlled substances, but because of the added burden of en- suring a law enforcement presence at take-back events, most programs are not currently accepting controlled substances from consumers. -

Enviroshopping1 Marie Hammer and Joan Papadi2
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. Fact Sheet HE 3167 Enviroshopping1 Marie Hammer and Joan Papadi2 You have tremendous influence in the board fossil-based fuels can also degrade the environment, rooms of American companies. They listen each time such as oil spills occurring in the ocean. And the you open your wallet. They will listen when you say production of fossil-based fuels affects the ‘‘Reduce, Reuse, Recycle.’’ They will listen when you environment, as when coal is strip-mined. ‘‘Reject and Respond.’’ As an enviroshopper, consider the environment when you compare the convenience, Hydro-electric power is not without its the cost and the quality of your purchases. You will environmental effects. To create the power source, feel proud that you did ‘‘the right thing’’ for yourself thousands of acres of land are dammed up and and future generations. flooded. As a result, the river ecology and wildlife habitat are destroyed. Nuclear power, too, produces Grains of sand make mighty sand dunes, and bits radio-active waste for which we have not yet found a of trash make mountains of garbage. In Florida we solution. throw away about eight pounds of garbage per person each day. The national average is about 3.5 pounds Most people are aware of the environmental per day. The Florida rate is increased somewhat by damage possible when disposing of waste by landfill the trash from our visitors (about 35 million each or burning. Few people realize that creating a product year). such as packaging from raw materials causes more environmental damage than disposing of it. -
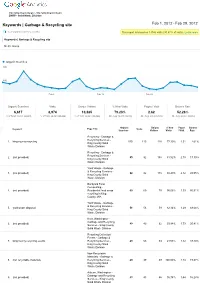
Keywords | Garbage & Recycling Site
http://www.kingcounty.gov http://www.kingcounty.gov DNRP Solid Waste Division Keywords | Garbage & Recycling site Feb 1, 2012 Feb 29, 2012 % of organic searches: 23.23% This report is based on 13346 visits (30.47% of visits). Learn more Keywords | Garbage & Recycling site Metric Group Organic Searches 800 400 Feb 8 Feb 15 Feb 22 Organic Searches Visits Unique Visitors % New Visits Pages / Visit Bounce Rate 6,617 8,974 10,385 79.23% 2.62 52.26% % of Total: 23.23% (28,481) % of Total: 20.49% (43,806) % of Total: 28.56% (36,365) Site Avg: 76.47% (3.61%) Site Avg: 2.95 (11.18%) Site Avg: 50.55% (3.39%) Organic Unique % New Pages Bounce Keyword Page Title Visits Searches Visitors Visits / Visit Rate Recycling Garbage & Recycling Services 1. king county recycling 115 115 128 77.39% 1.51 2.61% King County Solid Waste Division Recycling Garbage & Recycling Services 2. (not provided) 85 92 158 81.52% 2.78 17.39% King County Solid Waste Division Yard Waste Garbage & Recycling Services 3. (not provided) 82 82 118 80.49% 2.12 28.05% King County Solid Waste Division Backyard Food Composting 4. (not provided) Residential food scrap 69 69 79 95.65% 1.33 85.51% recycling in King County, WA Yard Waste Garbage & Recycling Services 5. yard waste disposal 56 56 59 82.14% 1.29 69.64% King County Solid Waste Division Kent, Washington Garbage and Recycling 6. (not provided) 49 49 62 93.88% 1.73 20.41% Services King County Solid Waste Division Recycling Collection Events Garbage & 7. -

Patient Options for Safe and Effective Disposal of Unused Opioids
United States Government Accountability Office Report to Congressional Committees September 2019 PRESCRIPTION OPIOIDS Patient Options for Safe and Effective Disposal of Unused Opioids GAO-19-650 September 2019 PRESCRIPTION OPIOIDS Patient Options for Safe and Effective Disposal of Unused Opioids Highlights of GAO-19-650, a report to congressional committees Why GAO Did This Study What GAO Found In 2017, an estimated 11.1 million The Food and Drug Administration (FDA), Drug Enforcement Administration Americans misused a prescription pain (DEA), and Environmental Protection Agency (EPA) recommend that patients reliever, which included opioids. This dispose of unused presciption opioids by bringing them to DEA-registered misuse contributes to opioid abuse and collection sites or a DEA take-back event, or using mail-back programs. As of death, which has quintupled from 1999 April 2019, 70 percent of the U.S. population lived less than 5 miles from to 2017; about 17,000 people died from permanent collection sites, which are often located at pharmacies. If collection prescription opioid overdoses in 2017. sites, take-back events, or mail-back programs are not feasible, FDA Government agencies and stakeholders recommends quickly and permanently removing the most dangerous prescription have attempted to address the potential opioids, such as hydrocodone and fentanyl, from the home by flushing them for misuse and abuse by facilitating safe down the toilet. For all other prescription opioids, the agencies recommend disposal of unused prescription opioids disposal in the trash after mixing them with unpalatable substances, such as cat and other drugs. litter. Commercial products to facilitate in-home disposal also exist, and FDA is The SUPPORT for Patients and aware that patients may opt to use these products for disposal in the trash. -

Overview of Eight Medicine Disposal Products
OVERVIEW OF EIGHT MEDICINE DISPOSAL PRODUCTS Community Environmental Health Strategies LLC April 21, 2017 for San Francisco Department of the Environment Executive Summary Secure disposal of leftover and expired medications is a key prevention strategy in reducing misuse and diversion of medicines to combat drug abuse and prevent overdoses. Proper disposal of leftover and expired medicines prevents those medicines from contributing to pharmaceutical pollution in waterways and water supplies. Disposing of unwanted medicines in the household trash creates risks of unintended exposures, drug diversion, and environmental pollution. This recognition has increased interest in and implementation of secure medicine take-back programs. It has also sparked a new market sector of products designed for in-home disposal of waste medicines that are described as easy and safe ways to alter pharmaceuticals so that they are safe for trash disposal. This report examines eight medicine disposal products based on information available from each manufacturer’s website, promotional materials, and product labels as of March 2017. Seven products marketed to consumers were reviewed: Deterra, DisposeRx, Drug Buster, Element MDS, Pill Catcher, Pill Terminator, and Rx Destroyer. These products recommend trash disposal of the product-pharmaceutical mixture and are also marketed for use by healthcare facilities and practitioners. Cactus Smart Sink was reviewed as a related product that is intended for use solely by healthcare facilities, with final disposal by incineration according to regulatory requirements. The medicine disposal products marketed for consumer use are provided in bottles or a pouch. Home users are instructed to add unwanted medicines in pill or liquid form to a stated capacity; some of the products also treat patches. -

Talking Trash: Oral Histories of Food In/Security from the Margins of a Dumpster By: Rachel A
Talking Trash: Oral Histories of Food In/Security from the Margins of a Dumpster By: Rachel A. Vaughn Submitted to the graduate degree program in American Studies and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Chairperson, Dr. Sherrie Tucker ________________________________ Dr Tanya Hart ________________________________ Dr. Sheyda Jahanbani ______________________________ Dr. Phaedra Pezzullo ________________________________ Dr. Ann Schofield Date Defended: Friday, December 2, 2011 The Dissertation Committee for Rachel A. Vaughn certifies that this is the approved version of the following dissertation: Talking Trash: Oral Histories of Food In/Security from the Margins of a Dumpster ________________________________ Chairperson, Dr. Sherrie Tucker Date approved: December, 2, 2011 ii Abstract This dissertation explores oral histories with dumpster divers of varying food security levels. The project draws from 15 oral history interviews selected from an 18-interview collection conducted between Spring 2008 and Summer 2010. Interviewees self-identified as divers; varied in economic, gender, sexual, and ethnic identity; and ranged in age from 18-64 years. To supplement this modest number of interviews, I also conducted 52 surveys in Summer 2010. I interview divers as theorists in their own right, and engage the specific ways in which the divers identify and construct their food choice actions in terms of individual food security and broader ecological implications of trash both as a food source and as an international residue of production, trade, consumption, and waste policy. This research raises inquiries into the gender, racial, and class dynamics of food policy, informal food economies, common pool resource usage, and embodied histories of public health and sanitation. -

Proper Disposal of Medications the Risks of Improper Disposal and the Benefits of Take-Back Programs
Proper Disposal of Medications The Risks of Improper Disposal and the Benefits of Take-Back Programs Tag Words: Medication; Disposal of Medications; Prescription Drugs; Over the Counter Drugs Authors: Brian Estrella, Minhazur Rahman, Kevin Ng with Julie M. Fagan, Ph.D. Summary Both prescription and non-prescription drugs are some of the most common items found in every American household. What many people fail to realize is that these medications have expiration dates and must be properly disposed of. As the use of medication becomes more prevalent in our society a major problem is occurring: what should a person do with expired medication? Recent trends in the United States have shown that children ages 12 to 17 are likely to abuse prescription drugs more than all other illicit drugs except marijuana. These same studies have shown that children in this age group are likely to obtain these drugs from the home. Other studies have been conducted, showing small amounts of antibiotics in groundwater. To combat these issues, the United States government has recently passed a law called The Secure and Responsible Drug Disposal Act. This law allows local pharmacies to take back prescription and over-the-counter drugs for proper disposal. Our goal for this project is to raise awareness among our friends, students, and peers about the dangers associated with improper medical waste disposal. We hope to accomplish this by writing to the corporate headquarters of local pharmacies, compiling this classepedia document, and through the production of documentary and instructional videos on how to properly and safely dispose of any drugs. -

SYMBIOSIS the Journal of Ecologically Sustainable Medicine
SYMBIOSIS The Journal of Ecologically Sustainable Medicine THE TELEOSIS INSTITUTE Vol. 4 No. 2 Spring/Summer 2007 In This Issue Letter from the Director Health News: Pharmaceutical Pollution Green Pharmacy Pharmaceutical Pollution: Ecology and Toxicology Christian Daughton and the Ecology of PPCPs Water Quality in the 21st Century The 4 Ts—Assessing Exposure to Multiple Chemicals Green Pharmacy: Preventing Pollution A Cross Sector Approach Ecological Economics and the Drug Life Cycle Unused and Expired Medicines Pollution Prevention Partners Spotlight on Green Pharmacy Website Review Book Review www.teleosis.org SYMBIOSIS Board of Directors George Brandt Treasurer, San Francisco, CA Wendy Buffet, MD Secretary, San Francisco, CA Sean Esbjorn-Hargens, PhD Sebastopol, CA Calista Hunter, MD San Ramon, CA Joel Kreisberg, DC Chairman, Berkeley, CA Advisory Board Bhaswati Bhattacharya, MD Director of Research Department of Medicine, Wyckoff Heights Medical Center Daniel Callahan, PhD Director of International Program, The Hastings Center Larry Dossey, MD Executive Editor, Explore: Journal of Science and Healing Gil Friend, MS President and CEO, Natural Logic, Inc. Constance Grauds, RPh President, Association of Natural Medicine Pharmacists Ben Kligler, MD Integrative Medicine, Continuum Center for Health and Healing, Beth Israel Hospital Lee Klinger, PhD Independent Scientist, The Sudden Oak Life Task Force Tara Levy, ND President, California Naturopathic Doctors Association David Orr, PhD Chair of the Environmental Studies Program, Oberlin -

Minutes Riley County Solid Waste Management Committee
MINUTES RILEY COUNTY SOLID WASTE MANAGEMENT COMMITTEE Thursday, March 1, 2018 Riley County Office Building 7:00 P.M. 2nd Floor Conference Room Members Present: Steve Galitzer, Leon Hobson, David Kreller, Perry Piper, Casey Smithson, William Spiegel, Monty Wedel, Ron Wells and Fran Zerby Others Present: Gary Rosewicz and Karen McCulloh Members Absent: Betty Book, William Clark, Dennis Peterson, Charly Pottorf, Dave Shover, Judy Willingham and Howard Wilson. Steve Galitzer, Chairman, called the meeting to order. Sign-in, Introductions The sign-in sheet and introductions were completed. Approve the minutes of the previous meeting David Kreller moved and William Spiegel seconded to approve the minutes of the March 2, 2017 meeting as presented. Motion approved. Update on Solid Waste budget Gary Rosewicz presented the attached “2018 Solid Waste Advisory Board Report”. He said with projected revenues and expenditures he doesn’t recommend any increases to the current rates for 2018. He did indicate that this is barring any unforeseen major expenditures but will still have to overlay the roadway and area around the station. Mr. Rosewicz did state that eventually the crane will have to be replaced. David Kreller asked if KDHE requires the county to have a reserve or bond. Gary Rosewicz replied the county is not required to have a reserve or bond but the county does have an insurance policy. He also said the Hamm’s contract will be expiring in 2020. These contracts in the past have been written to automatically annually renew for a 10 year period which helps to keep the bid costs lower.