MEDICINE DISPOSAL PRODUCTS an Overview of Products & Performance Questions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Navy DDG-51 and DDG-1000 Destroyer Programs: Background and Issues for Congress
Navy DDG-51 and DDG-1000 Destroyer Programs: Background and Issues for Congress Ronald O'Rourke Specialist in Naval Affairs February 3, 2012 Congressional Research Service 7-5700 www.crs.gov RL32109 CRS Report for Congress Prepared for Members and Committees of Congress Navy DDG-51 and DDG-1000 Destroyer Programs: Background and Issues for Congress Summary Procurement of Arleigh Burke (DDG-51) class Aegis destroyers resumed in FY2010 after a four- year hiatus. Congress funded the procurement of one DDG-51 in FY2010, two in FY2011, and one more in FY2012. The Navy’s FY2012 budget submission calls for procuring seven more in FY2013-FY2016. DDG-51s to be procured through FY2015 are to be of the current Flight IIA design. The Navy wants to begin procuring a new version of the DDG-51 design, called the Flight III design, starting in FY2016. The Flight III design is to feature a new and more capable radar called the Air and Missile Defense Radar (AMDR). The Navy began preliminary design work on the Flight III DDG-51 in FY2012. The Navy wants to use a multiyear procurement (MYP) contract for DDG- 51s to be procured from FY2013 through FY2017. The Navy’s proposed FY2012 budget requested $1,980.7 million in procurement funding for the DDG-51 planned for procurement in FY2012. This funding, together with $48.0 million in advance procurement funding provided in FY2011, would complete the ship’s total estimated procurement cost of $2,028.7 million. The Navy’s proposed FY2012 budget also requested $100.7 million in advance procurement funding for two DDG-51s planned for procurement in FY2013, $453.7 million in procurement funding to help complete procurement costs for the three Zumwalt (DDG-1000) class destroyers that were procured in FY2007 and FY2009, and $166.6 million in research and development funding for the AMDR. -

Sample Pages to Thor
1 only A Stranger Arrives The air was dry and cold near the small town of Puente Antiguo, New Mexico. Darcy Lewis sat in the driver’s seatReview of an old van full of expensive scientic equipment. She was a college student working with scientist Jane Foster. Another scientist, ForDr. Erik Selvig, sat in the passenger seat. In the back of the van, Jane- opened the roof and climbed onto it. Selvig followed her, and they looked up into the darkness. “So what exactly are we looking for?” Selvig asked. He was a friend of Jane’s father and knew her well. “Why did you ask me to join you here in New Mexico?” “I told youSample. Something strange is happening in the night sky and I need your help,” Jane replied. “It’s a little dierent each time. Once it was a pool of stars in a corner of the sky. But last week it was a rainbow—” A noise from her computer stopped her. “Wait for it!” she said excitedly. Nothing happened. The sky above them stayed calm and black. Darcy put her head out of the window and looked up at Jane. “Can I turn on the radio?” she asked. She sounded bored. “No!” Jane replied angrily. She and Selvig climbed back inside the van. “I don’t understand,” she said. She opened a small book and looked at her notes. She always carried this notebook—inside it was her life’s work. M01_THOR_SB3_GLB_05991.indd 1 3/6/18 1:53 PM 2 “These changes have happened many times, always at night, Erik!” She checked her computer. -

Fantastic Four Compendium
MA4 6889 Advanced Game Official Accessory The FANTASTIC FOUR™ Compendium by David E. Martin All Marvel characters and the distinctive likenesses thereof The names of characters used herein are fictitious and do are trademarks of the Marvel Entertainment Group, Inc. not refer to any person living or dead. Any descriptions MARVEL SUPER HEROES and MARVEL SUPER VILLAINS including similarities to persons living or dead are merely co- are trademarks of the Marvel Entertainment Group, Inc. incidental. PRODUCTS OF YOUR IMAGINATION and the ©Copyright 1987 Marvel Entertainment Group, Inc. All TSR logo are trademarks owned by TSR, Inc. Game Design Rights Reserved. Printed in USA. PDF version 1.0, 2000. ©1987 TSR, Inc. All Rights Reserved. Table of Contents Introduction . 2 A Brief History of the FANTASTIC FOUR . 2 The Fantastic Four . 3 Friends of the FF. 11 Races and Organizations . 25 Fiends and Foes . 38 Travel Guide . 76 Vehicles . 93 “From The Beginning Comes the End!” — A Fantastic Four Adventure . 96 Index. 102 This book is protected under the copyright laws of the United States of America. Any reproduction or other unauthorized use of the material or artwork contained herein is prohibited without the express written consent of TSR, Inc., and Marvel Entertainment Group, Inc. Distributed to the book trade in the United States by Random House, Inc., and in Canada by Random House of Canada, Ltd. Distributed to the toy and hobby trade by regional distributors. All characters appearing in this gamebook and the distinctive likenesses thereof are trademarks of the Marvel Entertainment Group, Inc. MARVEL SUPER HEROES and MARVEL SUPER VILLAINS are trademarks of the Marvel Entertainment Group, Inc. -

Legal Issues Relating to the Disposal of Dispensed Controlled Substances
. Legal Issues Relating to the Disposal of Dispensed Controlled Substances Brian T. Yeh Legislative Attorney July 30, 2010 Congressional Research Service 7-5700 www.crs.gov R40548 CRS Report for Congress Prepared for Members and Committees of Congress c11173008 . Legal Issues Relating to the Disposal of Dispensed Controlled Substances Summary According to the White House Office of National Drug Control Policy, the intentional use of prescription drugs for non-medical purposes is the fastest-growing drug problem in the country and the second-most common form of illicit drug abuse among teenagers in the United States, behind marijuana use. Young adults and teenagers may find their parents’ prescription drugs in unsecured medicine cabinets or other obvious locations in the home, or they may retrieve expired or unwanted medication from the trash. It is believed that properly disposing of unwanted medications would help prevent prescription drug abuse by reducing the accessibility and availability of such drugs. Yet throwing prescription medications into the trash or flushing them down the toilet may not be environmentally responsible. In response, many local communities and states have implemented pharmaceutical disposal programs (often referred to as drug “take- back” programs) that collect unused and unwanted medications from patients for incineration or other method of destruction that complies with federal and state laws and regulations, including those relating to public health and the environment. Prescription drugs may be categorized as either controlled substance medication or non- controlled substance medication. Pharmaceutical controlled substances, such as narcotic pain relievers OxyContin® and Vicodin®, are among the most commonly abused prescription drugs. -

Safe Disposal of Unused Controlled Substances
Safe Disposal of Unused Controlled Substances/ Current Challenges and Opportunities for Reform Prepared for: King Pharmaceuticals, Inc. Prepared by: Sharon Siler Suzanne Duda Ruth Brown Josette Gbemudu Scott Weier Jon Glaudemans King Pharmaceuticals, Inc. provided funding for this research. Avalere Health maintained full editorial control and the conclusions expressed here are those of the authors. TABLE OF CONTENTS 2 EXECUTIVE SUMMARY 6 INTRODUCTION 14 METHODOLOGY 18 CURRENT LANDSCAPE FOR DRUG DISPOSAL PROGRAMS 32 CRITICAL SUCCESS FACTORS 52 MODELS FOR REFORM 60 CONCLUSION 62 ACKNOWLEDGEMENTS 64 ENDNOTES King Pharmaceuticals, Inc. provided funding for this research. Avalere Health maintained full editorial control and the conclusions expressed here are those of the authors. EXECUTIVE SUMMARY SAFE DISPOSAL OF UNUSED CONTROLLED SUBSTANCES Disposal of unused prescription drugs, and controlled substances in particular, is a complicated issue. Unused drug take-back programs are emerging across the country as one strategy for reducing drug abuse, accidental poison- ing, and flushing drugs into the water supply. Current laws and regulations regarding controlled substances, however, limit these programs from accepting all drugs without strict oversight from law enforcement. SIGNIFICANT BARRIERS The Controlled Substances Act and Drug Enforcement Agency regulations dictate who can handle controlled substances, and are two of the most significant challenges today facing efforts to dispose of unused drugs. The law and regulations prohibit pharmacies, providers, and hospitals from collecting controlled substances that have already been dispensed to consumers. There is an exception that allows law enforcement officers to accept controlled substances, but because of the added burden of en- suring a law enforcement presence at take-back events, most programs are not currently accepting controlled substances from consumers. -

PDF Download Warriors 3 Pdf Free Download
WARRIORS 3 PDF, EPUB, EBOOK George R R Martin,Gardner Dozois | 360 pages | 02 Aug 2011 | St Martin's Press | 9780765360281 | English | New York, United States Warriors 3 PDF Book The two player mode new to Dynasty Warriors 3 allows players to either go head-to-head in one-on-one fights or play co-operatively in any of the stages. Science Fiction. He gave high praise to how much the game was based on the original Romance of the Three Kingdoms story and even went as far as saying "All the costuming of the 3D models is ethnically authentic and beautifully lavished" whereas a number of reviews described the graphical quality as being plain and blurry Such as the said IGN review. Diana Gabaldon Goodreads Author Contributor. Archived from the original PDF on April 21, In co-operative play the characters retain their saved statistics, there are no alterations to the stage and the players gain the ability to perform a more powerful version of their Musou attack Special Attack. The Warriors Three were a team of elite Asgardian warriors, comprised of some of the best warriors of Asgard. Other books in the series. Namespaces Article Talk. Smartphone :. Something like that doesn't belong in an anthology like this one, in my opinion. IGN strongly criticized the Xbox version over the sound, both music and the voice acting. Nice diverse collection of stories! Start Your Free Trial. Zhu Rong and Nu Wa are exceptions as Zhu Rong was said to be the only female to have fought in that era and Nu Wa's character is fictional and based on ancient myth. -
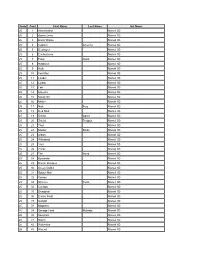
Form Card First Name Last Name Set Name 25 1 Abomination Marvel 3D
Form Card First Name Last Name Set Name 25 1 Abomination Marvel 3D 25 2 Baron Zemo Marvel 3D 25 3 Black Widow Marvel 3D 25 4 Captain America Marvel 3D 25 5 Destroyer Marvel 3D 25 6 Enchantress Marvel 3D 25 7 Frost Giant Marvel 3D 25 8 Hawkeye Marvel 3D 25 9 Hulk Marvel 3D 25 10 Iron Man Marvel 3D 25 11 Leader Marvel 3D 25 12 Lizard Marvel 3D 25 13 Loki Marvel 3D 25 14 Maestro Marvel 3D 25 15 Maria Hill Marvel 3D 25 16 Melter Marvel 3D 25 17 Nick Fury Marvel 3D 25 18 Red Skull Marvel 3D 25 19 Shield Agent Marvel 3D 25 20 Shield Trooper Marvel 3D 25 21 Thor Marvel 3D 25 22 Master Strike Marvel 3D 25 23 Ultron Marvel 3D 25 24 Whirlwind Marvel 3D 25 25 Ymir Marvel 3D 25 26 Zzzax Marvel 3D 25 27 The Hand Marvel 3D 25 28 Bystander Marvel 3D 25 29 Doctor Octopus Marvel 3D 25 30 Green Goblin Marvel 3D 25 31 Spider-Man Marvel 3D 25 32 Venom Marvel 3D 25 33 Scheme Twist Marvel 3D 25 34 Cyclops Marvel 3D 25 35 Deadpool Marvel 3D 25 36 Emma Frost Marvel 3D 25 37 Gambit Marvel 3D 25 38 Magneto Marvel 3D 25 39 Savage Land Mutates Marvel 3D 25 40 Sentinels Marvel 3D 25 41 Storm Marvel 3D 25 42 Wolverine Marvel 3D 25 43 Wound Marvel 3D 25 44 Egghead Marvel 3D 25 45 Daredevil Marvel 3D 25 46 Elektra Marvel 3D 25 47 Gladiator Marvel 3D 25 48 Punisher Marvel 3D 25 49 Blade Marvel 3D 25 50 Daredevil Marvel 3D 25 51 Elektra Marvel 3D 25 52 Ghost Rider Marvel 3D 25 53 Iron Fist Marvel 3D 25 54 Punisher Marvel 3D 25 55 Blade Marvel 3D 25 56 Daredevil Marvel 3D 25 57 Ghost Rider Marvel 3D 25 58 Iron Fist Marvel 3D 25 59 Punisher Marvel 3D 25 60 Electro Marvel -

Patient Options for Safe and Effective Disposal of Unused Opioids
United States Government Accountability Office Report to Congressional Committees September 2019 PRESCRIPTION OPIOIDS Patient Options for Safe and Effective Disposal of Unused Opioids GAO-19-650 September 2019 PRESCRIPTION OPIOIDS Patient Options for Safe and Effective Disposal of Unused Opioids Highlights of GAO-19-650, a report to congressional committees Why GAO Did This Study What GAO Found In 2017, an estimated 11.1 million The Food and Drug Administration (FDA), Drug Enforcement Administration Americans misused a prescription pain (DEA), and Environmental Protection Agency (EPA) recommend that patients reliever, which included opioids. This dispose of unused presciption opioids by bringing them to DEA-registered misuse contributes to opioid abuse and collection sites or a DEA take-back event, or using mail-back programs. As of death, which has quintupled from 1999 April 2019, 70 percent of the U.S. population lived less than 5 miles from to 2017; about 17,000 people died from permanent collection sites, which are often located at pharmacies. If collection prescription opioid overdoses in 2017. sites, take-back events, or mail-back programs are not feasible, FDA Government agencies and stakeholders recommends quickly and permanently removing the most dangerous prescription have attempted to address the potential opioids, such as hydrocodone and fentanyl, from the home by flushing them for misuse and abuse by facilitating safe down the toilet. For all other prescription opioids, the agencies recommend disposal of unused prescription opioids disposal in the trash after mixing them with unpalatable substances, such as cat and other drugs. litter. Commercial products to facilitate in-home disposal also exist, and FDA is The SUPPORT for Patients and aware that patients may opt to use these products for disposal in the trash. -

Overview of Eight Medicine Disposal Products
OVERVIEW OF EIGHT MEDICINE DISPOSAL PRODUCTS Community Environmental Health Strategies LLC April 21, 2017 for San Francisco Department of the Environment Executive Summary Secure disposal of leftover and expired medications is a key prevention strategy in reducing misuse and diversion of medicines to combat drug abuse and prevent overdoses. Proper disposal of leftover and expired medicines prevents those medicines from contributing to pharmaceutical pollution in waterways and water supplies. Disposing of unwanted medicines in the household trash creates risks of unintended exposures, drug diversion, and environmental pollution. This recognition has increased interest in and implementation of secure medicine take-back programs. It has also sparked a new market sector of products designed for in-home disposal of waste medicines that are described as easy and safe ways to alter pharmaceuticals so that they are safe for trash disposal. This report examines eight medicine disposal products based on information available from each manufacturer’s website, promotional materials, and product labels as of March 2017. Seven products marketed to consumers were reviewed: Deterra, DisposeRx, Drug Buster, Element MDS, Pill Catcher, Pill Terminator, and Rx Destroyer. These products recommend trash disposal of the product-pharmaceutical mixture and are also marketed for use by healthcare facilities and practitioners. Cactus Smart Sink was reviewed as a related product that is intended for use solely by healthcare facilities, with final disposal by incineration according to regulatory requirements. The medicine disposal products marketed for consumer use are provided in bottles or a pouch. Home users are instructed to add unwanted medicines in pill or liquid form to a stated capacity; some of the products also treat patches. -

AFFILIATION LIST • Black Panther • Black Widow Below You Will Find a List of All Current Affiliations Cards and Characters on Them
AFFILIATIONS 1/08/21 AFFILIATION LIST • Black Panther • Black Widow Below you will find a list of all current affiliations cards and characters on them. As more characters are added to the game • Black Widow, Agent of S.H.I.E.L.D. this list will be updated. A-FORCE • Captain Marvel • Hawkeye • She-Hulk (k) • Hulk • Angela • Iron Man • Black Widow • She-Hulk • Black Widow, Agent of Shield • Thor, Prince of Asgard • Captain Marvel • Vision • Crystal • Wasp • Domino • Wolverine • Gamora BLACK ORDER • Medusa • Thanos, The Mad Titan (k) • Okoye • Black Dwarf • Scarlet Witch • Corvus Glaive • Shuri • Ebony Maw • Storm • Proxima Midnight • Valkyrie BROTHERHOOD OF MUTANTS • Wasp k ASGARD • Magneto ( ) • Mystique (k) • Thor, Prince of Asgard (k) • Juggernaut • Angela • Quicksilver • Enchantress • Sabretooth • Hela, Queen of Hel • Scarlet Witch • Loki, God of Mischief • Toad • Valkyrie AVENGERS • Captain America (k) • Ant-Man • Beast Atomic Mass Games and logo are TM of Atomic Mass Games. Atomic Mass Games, 1995 County Road B2 W, Roseville, MN, 55113, USA, 1-651-639-1905. © 2021 MARVEL Actual components may vary from those shown. CABAL DEFENDERS • Red Skull (k) • Doctor Strange (k) • Baron Zemo • Daredevil • Bullseye • Ghost Rider • Crossbones • Hawkeye • Enchantress • Hulk • Killmonger • Iron Fist • Kingpin • Luke Cage • Loki, God of Mischief • Spider-Man (Peter Parker) • Magneto • Valkyrie • M.O.D.O.K. • Wolverine • Mystique • Wong • Sabretooth WAKANDA • Ultron • Black Panther (k) CRIMINAL SYNDICATE • Killmonger • Kingpin (k) • Okoye • Black Cat • Shuri • Bullseye • Storm • Crossbones GUARDIANS OF THE GALAXY • Green Goblin • Star-Lord (k) • Killmonger • Angela • M.O.D.O.K. • Drax the Destroyer • Mysterio • Gamora • Taskmaster • Groot • Nebula • Rocket Raccoon • Ronan the Accuser Atomic Mass Games and logo are TM of Atomic Mass Games. -

Editor in Chiefaxel Alonso Chief Creative Officer Joe
CHAD BOWERS & CHRIS SIMS ALTI FIRMANSYAH MATT MILLA VC’s TRAVIS LANHAM WRITERS ART COLOR ART LETTERING DAVID NAKAYAMA CARLOS LAO HEATHER ANTOS & JORDAN D. WHITE COVER ART PRODUCTION DESIGN EDITORS EDITOR IN CHIEF AXEL ALONSO CHIEF CREATIVE OFFICER JOE QUESADA PUBLISHER DAN BUCKLEY EXECUTIVE PRODUCER ALAN FINE X-MEN CREATED BY STAN LEE & JACK KIRBY X-MEN ‘92 No. 9, January 2017. Published Monthly by MARVEL WORLDWIDE, INC., a subsidiary of MARVEL ENTERTAINMENT, LLC. OFFICE OF PUBLICATION: 135 West 50th Street, New York, NY 10020. BULK MAIL POSTAGE PAID AT NEW YORK, NY AND AT ADDITIONAL MAILING OFFICES. © 2016 MARVEL No similarity between any of the names, characters, persons, and/or institutions in this magazine with those of any living or dead person or institution is intended, and any such similarity which may exist is purely coincidental. $3.99 per copy in the U.S. (GST #R127032852) in the direct market; Canadian Agreement #40668537. Printed in the USA. Subscription rate (U.S. dollars) for 12 issues: U.S. $26.99; Canada $42.99; Foreign $42.99. POSTMASTER: SEND ALL ADDRESS CHANGES TO X-MEN ‘92, C/O MARVEL SUBSCRIPTIONS P.O. BOX 727 NEW HYDE PARK, NY 11040. TELEPHONE # (888) 511-5480. FAX # (347) 537-2649. subscrip- [email protected]. ALAN FINE, President, Marvel Entertainment; DAN BUCKLEY, President, TV, Publishing & Brand Management; JOE QUESADA, Chief Creative Officer; TOM BREVOORT, SVP of Publishing; DAVID BOGART, SVP of Business Affairs & Operations, Publishing & Partnership; C.B. CEBULSKI, VP of Brand Management & Development, Asia; DAVID GABRIEL, SVP of Sales & Marketing, Publishing; JEFF YOUNGQUIST, VP of Production & Special Projects; DAN CARR, Executive Director of Publishing Technology; ALEX MORALES, Director of Publishing Operations; SUSAN CRESPI, Production Manager; STAN LEE, Chairman Emeritus. -

Childhood Idols, Shifting from Superheroes to Public Health Heroes
Journal of Public Health Advance Access published February 16, 2015 Journal of Public Health | pp. 1–5 | doi:10.1093/pubmed/fdv013 Childhood idols, shifting from superheroes to public health heroes Brandon Brown1, Melissa Nasiruddin2, Alejandra Cabral1, Melissa Soohoo1 1Program in Public Health, University of California, Irvine, CA 92697-3957, USA 2Midwestern University College of Osteopathic Medicine, Glendale, AZ, USA Address correspondence to Brandon Brown, E-mail: [email protected] ABSTRACT It is early Saturday morning: a day for heroes. Bogged down with various costumes, capes and action figures, young Nikoli bounds downstairs to catch reruns of Teen Titans. He puts his newly acquired reading skills to work, studying comic books and recreating the adventures therein. Nikoli imagines himself as the hero in his comics, defeating villains and saving victims, imitating the poses and catchphrases in the mirror. Although children like Nikoli will never gain super strength or the ability to fly, the superheroes they emulate in play are examples of people they can aspire to be. They don’t even have to be fictional heroes—if we widen the scope of children’s superheroes to include those that address real-life issues, or even real-life heroes who have made breakthroughs in fields such as public health, the impact could be tremendous. Imagine a world where Superman is mentioned in the same breath as Ignaz Semmelweis, the man who revolutionized sanitation in health care by demonstrating that hand washing prevents the spread of infection. Children who idolize the champions of health care could someday grow up to be heroes themselves, fighting epidemics and saving lives through education, treatment and research.