Marine Resource Management for Misali Island 87
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Evaluation of Environmental Education Programs on Pemba Island Lindsay Kingston SIT Study Abroad
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Spring 2013 An Evaluation of Environmental Education Programs on Pemba Island Lindsay Kingston SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Growth and Development Commons, Natural Resource Economics Commons, Natural Resources Management and Policy Commons, and the Sustainability Commons Recommended Citation Kingston, Lindsay, "An Evaluation of Environmental Education Programs on Pemba Island" (2013). Independent Study Project (ISP) Collection. 1523. https://digitalcollections.sit.edu/isp_collection/1523 This Unpublished Paper is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. An Evaluation of Environmental Education Programs on Pemba Island Lindsay Kingston Middlebury College SIT Zanzibar, Tanzania Spring 2013 Advisor: Hamza Rijaal Academic Director: Nat Quansah Table of Contents Acknowledgements ……………………………………………………. 1 Abstract………………………………………………………………… 2 Introduction…………………………………………………………….. 2 Study Sites……………………………………………………………... 8 Methodology………………………………………………………….. 10 Limitations……………………………………………………………. 13 Results………………………………………………………………... 14 Discussion……………………………………………………………. 28 Conclusion…………………………………………………………… 35 Recommendations……………………………………………………. 36 Works Cited ………………………………………………………….. 37 Appendices …………………………………………………………... 39 Acknowledgements: I would like to thank Dr. Nat Quanash for his insight and advice from the beginning of the semester all the way to the end. A big thank you goes to Said for having answers to all my questions, solutions to all my problems and everlasting patience with not only me, but with every single student that has gone through this program. He is truly amazing. -

Kutafuta Maisha: Seeking a Life
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by NORA - Norwegian Open Research Archives Kutafuta Maisha: Seeking a Life Political Disengagement as a Life-Improving Strategy – a Study of Youths in Politically Polarized Zanzibar Kristin Birkeland Galteland Masteroppgave ved Sosialantropologisk institutt UNIVERSITETET I OSLO 20. mai 2010 2 Kutafuta Maisha: Seeking a Life Political Disengagement as a Life-Improving Strategy – a Study of Youths in Politically Polarized Zanzibar Kristin Birkeland Galteland 3 © Kristin Birkeland Galteland 2010 Kutafuta Maisha: Seeking a Life Political Disengagement as a Life-Improving Strategy - a Study of Youths in Politically Polarized Zanzibar Kristin Birkeland Galteland http://www.duo.uio.no/ Trykk: Reprosentralen, Universitetet i Oslo 4 Summary Youths in Zanzibar live under tough socio-economic conditions. As they follow developments in rural mainland Tanzania, and in other countries, they develop notions of what “the ideal” life different from previous generations’. Youths tend to not consider farming and fishing as real jobs anymore, rather seeking jobs in offices and business. In Zanzibar, it is believed that supporters of the ruling party have greater economic opportunities. For decades, Pemba Island has been the stronghold of the political opposition. Young Pembans therefore find it hard to seek the privileges of ruling party supporters. As there is little room in their community for critique of the opposition party, they seek to become apolitical through political disengagement. This is a difficult project, since the opposition party over time has become a central part of the people’s shared identity. This master thesis discusses everyday forms of resistance in Wete town on Pemba Island, where politics are strongly polarized. -

Kutafuta Maisha: Seeking a Life
Kutafuta Maisha: Seeking a Life Political Disengagement as a Life-Improving Strategy – a Study of Youths in Politically Polarized Zanzibar Kristin Birkeland Galteland Masteroppgave ved Sosialantropologisk institutt UNIVERSITETET I OSLO 20. mai 2010 2 Kutafuta Maisha: Seeking a Life Political Disengagement as a Life-Improving Strategy – a Study of Youths in Politically Polarized Zanzibar Kristin Birkeland Galteland 3 © Kristin Birkeland Galteland 2010 Kutafuta Maisha: Seeking a Life Political Disengagement as a Life-Improving Strategy - a Study of Youths in Politically Polarized Zanzibar Kristin Birkeland Galteland http://www.duo.uio.no/ Trykk: Reprosentralen, Universitetet i Oslo 4 Summary Youths in Zanzibar live under tough socio-economic conditions. As they follow developments in rural mainland Tanzania, and in other countries, they develop notions of what “the ideal” life different from previous generations’. Youths tend to not consider farming and fishing as real jobs anymore, rather seeking jobs in offices and business. In Zanzibar, it is believed that supporters of the ruling party have greater economic opportunities. For decades, Pemba Island has been the stronghold of the political opposition. Young Pembans therefore find it hard to seek the privileges of ruling party supporters. As there is little room in their community for critique of the opposition party, they seek to become apolitical through political disengagement. This is a difficult project, since the opposition party over time has become a central part of the people’s shared identity. This master thesis discusses everyday forms of resistance in Wete town on Pemba Island, where politics are strongly polarized. The thesis is also a discussion on collective memory, and notions of differences between ethnic and political groups. -

A Status Update on the Pemba Flying Fox Hannah “Hawa” Grose SIT Study Abroad
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Fall 2012 Who Else Would Plant The rT ees? A Status Update on the Pemba Flying Fox Hannah “Hawa” Grose SIT Study Abroad Catherine Grace “Rahma” Clemmens SIT Graduate Institute - Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Natural Resources and Conservation Commons, and the Population Biology Commons Recommended Citation Grose, Hannah “Hawa” and Clemmens, Catherine Grace “Rahma”, "Who Else Would Plant The rT ees? A Status Update on the Pemba Flying Fox" (2012). Independent Study Project (ISP) Collection. 1419. https://digitalcollections.sit.edu/isp_collection/1419 This Unpublished Paper is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. Who Else Would Plant The Trees? A Status Update on the Pemba Flying Fox Hannah “Hawa” Grose and Catherine Grace “Rahma” Clemmens Fall 2012 Clemmens&Grose1 Table of Contents Acknowledgements………………………………………………………………………1 Abstract……………………………………………………………………………………3 Introduction……………………………………………………………………………….4 Study Area…………………………………………………………………………………7 Methods……………………………………………………………………………….....10 Results…………………………………………………………………………………....11 Discussion………………………………………………………………………………..20 -

'From Stinkibar to Zanzibar': Disease, Medicine and Public Health in Colonial Urban Zanzibar, 1870-1963
'FROM STINKIBAR TO ZANZIBAR': DISEASE, MEDICINE AND PUBLIC HEALTH IN COLONIAL URBAN ZANZIBAR, 1870-1963 AMINAAMEIRISSA A Dissertation Submitted in Fulfillment of the Requirements for the Degree of Doctor of Philosophy in History at the University of KwaZulu-Natal May 2009 DECLARATION I declare that this dissertation has not been submitted to any other university and that it is my entirely own work that I have given due acknowledgement of all sources. CMX& AminHLa Ameir Issa rofessor Julie Parle 3 JMri£20pC{ 6s}\MAl lOOf ii DEDICATION I dedicate this dissertation to my children Abdul-Aziz, Nusayba, Atifa and Mahmoud And to my late father Ameir Issa Haji (1939-1995) TABLE OF CONTENTS ABSTRACT viii ACKNOWLEDGEMENT ix ILLUSTRATIONS xi ABBREVIATIONS xii CHRONOLOGY OF THE OMANI RULERS, ZANZIBAR xiii BRITISH ADMINISTRATORS, ZANZIBAR xiv GLOSSARY xv INTRODUCTION Disease, Medicine and Health, 1870-1963 1 Introduction 1 The Literature Review 9 Theoretical Framework 19 Sources and Structure 24 CHAPTER 1 A History of Disease in Urban Zanzibar, c. 1860s - c. 1870s 31 The Entrepot 31 "Stinky Town" 35 Endemic Diseases in Urban Zanzibar: Fever, Yaws and Elephantiasis 43 Epidemic Diseases: Smallpox, Cholera and Dengue fever 54 Disease, Death and Demography 64 Conclusion: Zanzibar Town in the mid-1800s, A Repository of Disease and Death 67 iv CHAPTER 2 The Arrival and Consolidation of Western Medicine in Urban Zanzibar, 1830-1889 68 The Arrival of Western Medicine in Urban Zanzibar 68 Consulate Physicians and Peoples' Illnesses 73 Cure and Christianity: -

Coastal Profile for Zanzibar 2014 Portfolio of Actions - Volumev Final Draft
Coastal Profile for Zanzibar 2014 Portfolio of Actions - VolumeV Final Draft Investment Prioritisation for Resilient Livelihoods and Ecosystems in Coastal Zones of Tanzania List of Contents List of Contents ........................................................................................................................................ iii List of Tables ............................................................................................................................................. v List of Figures ............................................................................................................................................ v Acronyms ................................................................................................................................................ vii Table of Units ........................................................................................................................................... xi INTRODUCTION ..................................................................................................................................... 1 Tanzania Coastal Zone ........................................................................................................................ 1 The Project ............................................................................................................................................. 1 Partners ................................................................................................................................................. -

Comprehensive Mult Year Plan
REVOLUTIONARY GOVERNMENT OF ZANZIBAR. COMPREHENSIVE MULT YEAR PLAN - ZANZIBAR 2010 – 2014 EPI/MOHSW ZANZIBAR July 2009. TABLE OF CONTENTS Acronyms And Abbreviations 3 Executive Summary 4 1.0 Background 4 1.1. Introduction .........................................................................................................................................4 1.2. Location And Surface Area............................................................................................................5 1.3 Administrative Structure ...............................................................................................................5 1.4 Climate....................................................................................................................................................5 1.5 Population Projection 2008..........................................................................................................5 1.6 Economy.................................................................................................................................................6 1.7 Socio-Economic Status .................................................. Error! Bookmark not defined. 1.8 Health Service Delivery...................................................................................................................7 1.9 Linkage Of Epi To Moh ...................................................................................................................8 1.10 Number Of Health Facilities By Districts................................................................................8 -
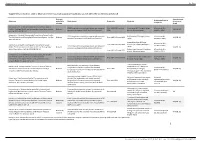
Supplementary Material, Table 1: Descriptive Information on Population-Based Data Sources Identified by the Literature Search
Supplementary material Inj Prev Supplementary material, table 1: Descriptive information on population-based data sources identified by the literature search Relevant, Ascertainment Study population & Reference potentially Study design Study date Study site of causes of sample size relevant death Kishamawe et al. Health & Demographic Surveillance System HDSS (longitudinal surveillance system with repeated From 1994. VA from Feb Kisesa ward (all 7 villages), Magu All residents (N = Profile: The Magu Health and Demographic Surveillance System. Relevant VA (ICD-10) censuses, continuous birth & death notifications) 1995. district, Mwanza region 35,569 in 2014) Int J Epi 2015; 44:1851-61. Mrema et al. Health & Demographic Surveillance System Profile: HDSS (longitudinal surveillance system with repeated Rufiji district (38 villages), Pwani All residents (N = The Rufiji Health and Demographic Surveillance System. Int J Epi Relevant From 1998. VA from 1999 VA (ICD-10) censuses, continuous birth & death notifications) region 103,503 in 2012) 2015; 44: 472-83. Across Kilombero & Ulanga All residents (N = Geubbels et al. Health & Demographic Surveillance System From 1996. VA from 2000 districts (25 villages), Morogoro HDSS (longitudinal surveillance system with repeated 126,836 in 2012) Profile: The Ifakara Rural and Urban Health and Demographic Relevant region VA (ICD-10) censuses, continuous birth & death notifications) Surveillance System. Int J Epi 2015; 44:848-61 Ifakara town (5 areas), Kilombero All residents (N = From 2007. VA from 2007 district, Morogoro region 44,992 in 2012) Kamugisha et al. Establishing and implementing Demographic Surveillance System as a tool for monitoring health HDSS (longitudinal surveillance system with repeated Korogwe district (14 villages across All residents (N = Relevant From 2005. -

Management of Programmes for Communicable Diseases Control in Sub-Saharan Africa
Management of Programmes for Communicable Diseases Control in Sub-Saharan Africa MPCDC 2010 PUBLIC HEALTH LABORATORY IVO DE CARNERI, PEMBA, ZANZIBAR 26th JULY – 6th AUGUST 2010 Seventh Edition In the process of obtaining the patronages from “Università degli Studi di Brescia - Who Collaborating Center on the implementation of TB/HIV collaborative activities and “Cooperazione Italiana allo Sviluppo”. 1 Table of contents Table of contents ............................................................................................................................................ 2 Welcome Message ......................................................................................................................................... 3 Secretariats and Lecturers ............................................................................................................................. 4 The Organizers .............................................................................................................................................. 5 Course introduction ........................................................................................................................................ 6 Course Announcement: MPCDC at a Glance ............................................................................................... 7 The MPCDC Scholarship ............................................................................................................................... 8 The MPCDC Venue ...................................................................................................................................... -

Project Documentaries
PROJECT/PROGRAMME PROPOSAL TO THE ADAPTATION FUND PART I:PROJECT/PROGRAMMEINFORMATION PART I: PROJECT/PROGRAMMEINFORMATION Project/Programme Category: Regular Project Title of Project/Programme: Enhancing Climate Change Resilience of Coastal Communities of Zanzibar Type of Implementing Entity: National Implementing Entity (NIE) Implementing Entity: Do not fill Executing Entity/ies: Department of Environment, Second Vice President’s Office, Zanzibar Amount of Financing Requested: US$ 1,000,000 1.0 Project Background and Context Zanzibar is one of the two countries that form the United Republic of Tanzania (URT). Located in the Indian Ocean, just south of the Equator, the Zanzibar archipelago comprises two major islands - Unguja and Pemba - and more than 50 other small and remote islets. In the last census, of 2012, Zanzibar had 1,303,569 inhabitants. The population had increased by 33% since the previous census of 2002, with an average annual growth rate of 2.8. Population growth rates are projected to be high. While Zanzibar is working towards alleviating abject poverty, climate change is yet another bottleneck to its socio.- economic development. Climate variability has caused prolonged dry periods and unpredictable rainfall patterns making crop cultivation unproductive. Freshwater resources are also in limited supply mainly dependent on seasonal rains that store water in inefficient groundwater aquifers consisting of freshwater lenses floating on the underlying seawater1.Furthermore, increasing temperatures have occasionally caused seal level rise leading to saltwater intrusion in low-lying farm fields, notably rice farms. To this end, the Revolutionary Government of Zanzibar in consultation with stakeholders and guided by Zanzibar’s development Vision 2020 and the MKUZA-III development plans, has developed a Zanzibar Climate Change Strategy (ZCCS) in 2014. -

The Revolutionary Government of Zanzibar
The Revolutionary Government of Zanzibar Ministry of Education and Vocational Training and President’s Office – Regional Administration and Local Government and Special Departments Annual Joint Education Sector Review Report 2019 Prepared by: Annual Joint Education Sector Review Technical Working Group March 2019 1 Foreword The purpose of this document is to outline a series of actionable recommendations that have come off the back of MoEVT and PO-RALGSD 2019 AJESR Meeting and Joint Field Visits carried out in February 2019. These recommendations have been formed from insights gathered during the field visits, presentations delivered at the AJESR meeting, group discussion and feedback from participants. The recommendations are intended to guide the Ministry’s strategic priorities over the coming year; in particular, they should influence the MTEF budgeting cycle due to begin in March 2019. In addition to this, this report also intends to summarize the key themes that emerged during the presentations at the AJESR meeting. The report also contains recommendations on how to improve the logistics of the AJESR meeting and field visits for next year. This document is intended primarily for MoEVT and PO-RALGSD policymakers, Development Partners and Civil Society Organizations in order to influence their decision-making ahead of the budgeting process for 2019/2020. It should also be disseminated to broader stakeholders in the Zanzibar education sector. The recommendations outlined in this report should be monitored and assessed throughout the year to ensure their timely implementation and enable the quick resolution of issues. At the AJESR meeting in 2020, the Ministry’s progress in implementing these recommendations will be assessed and reported on. -

From Stinkibar to Zanzibar’: Disease, Medicine and Public Health in Colonial Urban Zanzibar, 1870-1963
‘FROM STINKIBAR TO ZANZIBAR’: DISEASE, MEDICINE AND PUBLIC HEALTH IN COLONIAL URBAN ZANZIBAR, 1870-1963 AMINA AMEIR ISSALIBRARY - A Dissertation Submitted in Fulfillment of the Requirements for the Degree of Doctor of Philosophy in History at the University of KwaZulu-Natal CODESRIA May 2009 DECLARATION I declare that this dissertation has not been submitted to any other university and that it is my entirely own work that I have given due acknowledgement of all sources. LIBRARY - ______________________ ________________ Amina Ameir Issa Professor Julie Parle ________________ ________________ CODESRIA ii DEDICATION I dedicate this dissertation to my children Abdul-Aziz, Nusayba, Atifa and Mahmoud And to my late father Ameir Issa Haji (1939-1995) LIBRARY - CODESRIA iii TABLE OF CONTENTS ABSTRACT viii ACKNOWLEDGEMENT ix ILLUSTRATIONS xi ABBREVIATIONS xii CHRONOLOGY OF THE OMANI RULERS, ZANZIBAR xiii BRITISH ADMINISTRATORS, ZANZIBAR xiv GLOSSARY xv INTRODUCTION LIBRARY Disease, Medicine and Health, 1870-1963 - 1 Introduction 1 The Literature Review 9 Theoretical Framework 19 Sources and Structure 24 CHAPTER CODESRIA1 A History of Disease in Urban Zanzibar, c. 1860s – c. 1870s 31 The Entrepôt 31 “Stinky Town” 35 Endemic Diseases in Urban Zanzibar: Fever, Yaws and Elephantiasis 43 Epidemic Diseases: Smallpox, Cholera and Dengue fever 54 Disease, Death and Demography 64 Conclusion: Zanzibar Town in the mid-1800s, A Repository of Disease and Death 67 iv CHAPTER 2 The Arrival and Consolidation of Western Medicine in Urban Zanzibar, 1830-1889 68