Plant Speciation Across Environmental Gradients and the Occurrence and Nature of Hybrid Zones
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Speciation in Heliconius Butterflies: Minimal Contact Followed 2 by Millions of Generations of Hybridisation 3 Simon H
bioRxiv preprint doi: https://doi.org/10.1101/015800; this version posted March 2, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 1 Speciation in Heliconius Butterflies: Minimal Contact Followed 2 by Millions of Generations of Hybridisation 3 Simon H. Martin*1, Anders Eriksson*1,2, Krzysztof M. Kozak1, Andrea Manica1 and 4 Chris D. Jiggins1 5 1 Department of Zoology, University of Cambridge, Downing Street, Cambridge, CB2 6 3EJ, United Kingdom 7 2 Integrative Systems Biology Laboratory, King Abdullah University of Science 8 and Technology, Thuwal 23955-6900, Kingdom of Saudi Arabia 9 * These authors contributed equally 10 Corresponding Author: S.H. Martin, [email protected] 11 Running Head: Change in the rate of gene flow during butterfly speciation bioRxiv preprint doi: https://doi.org/10.1101/015800; this version posted March 2, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 2 12 Abstract 13 Documenting the full extent of gene flow during speciation poses a challenge, as 14 species ranges change over time and current rates of hybridisation might not reflect 15 historical trends. Theoretical work has emphasized the potential for speciation in the 16 face of ongoing hybridisation, and the genetic mechanisms that might facilitate this 17 process. -

Can Secondary Contact Following Range Expansion Be Distinguished from Barriers to Gene flow?
Can secondary contact following range expansion be distinguished from barriers to gene flow? Johanna Bertl1,2, Harald Ringbauer3,4 and Michael G.B. Blum5 1 Department of Molecular Medicine, Aarhus University, Aarhus, Denmark 2 Vienna Graduate School of Population Genetics, Vetmeduni Vienna, Vienna, Austria 3 Department of Human Genetics, University of Chicago, Chicago, IL, USA 4 Institute of Science and Technology Austria, Klosterneuburg, Austria 5 Laboratoire TIMC-IMAG, UMR 5525, Université Grenoble Alpes, CNRS, Grenoble, France ABSTRACT Secondary contact is the reestablishment of gene flow between sister populations that have diverged. For instance, at the end of the Quaternary glaciations in Europe, secondary contact occurred during the northward expansion of the populations which had found refugia in the southern peninsulas. With the advent of multi-locus markers, secondary contact can be investigated using various molecular signatures including gradients of allele frequency, admixture clines, and local increase of genetic differentiation. We use coalescent simulations to investigate if molecular data provide enough information to distinguish between secondary contact following range expansion and an alternative evolutionary scenario consisting of a barrier to gene flow in an isolation-by-distance model. We find that an excess of linkage disequilibrium and of genetic diversity at the suture zone is a unique signature of secondary contact. We also find that the directionality index c, which was proposed to study range expansion, is informative to distinguish between the two hypotheses. However, although evidence for secondary contact is usually conveyed by statistics related to admixture coefficients, we find that they can be confounded by isolation-by-distance. We recommend to account for the spatial repartition of fl Submitted 29 November 2016 individuals when investigating secondary contact in order to better re ect the Accepted 1 July 2018 complex spatio-temporal evolution of populations and species. -

Microevolution: Species Concept Core Course: ZOOL3014 B.Sc. (Hons’): Vith Semester
Microevolution: Species concept Core course: ZOOL3014 B.Sc. (Hons’): VIth Semester Prof. Pranveer Singh Clines A cline is a geographic gradient in the frequency of a gene, or in the average value of a character Clines can arise for different reasons: • Natural selection favors a slightly different form along the gradient • It can also arise if two forms are adapted to different environments separated in space and migration (gene flow) takes place between them Term coined by Julian Huxley in 1838 Geographic variation normally exists in the form of a continuous cline A sudden change in gene or character frequency is called a stepped cline An important type of stepped cline is a hybrid zone, an area of contact between two different forms of a species at which hybridization takes place Drivers and evolution of clines Two populations with individuals moving between the populations to demonstrate gene flow Development of clines 1. Primary differentiation / Primary contact / Primary intergradation Primary differentiation is demonstrated using the peppered moth as an example, with a change in an environmental variable such as sooty coverage of trees imposing a selective pressure on a previously uniformly coloured moth population This causes the frequency of melanic morphs to increase the more soot there is on vegetation 2. Secondary contact / Secondary intergradation / Secondary introgression Secondary contact between two previously isolated populations Two previously isolated populations establish contact and therefore gene flow, creating an -

Coupling, Reinforcement, and Speciation Roger Butlin, Carole Smadja
Coupling, Reinforcement, and Speciation Roger Butlin, Carole Smadja To cite this version: Roger Butlin, Carole Smadja. Coupling, Reinforcement, and Speciation. American Naturalist, Uni- versity of Chicago Press, 2018, 191 (2), pp.155-172. 10.1086/695136. hal-01945350 HAL Id: hal-01945350 https://hal.archives-ouvertes.fr/hal-01945350 Submitted on 5 Dec 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License vol. 191, no. 2 the american naturalist february 2018 Synthesis Coupling, Reinforcement, and Speciation Roger K. Butlin1,2,* and Carole M. Smadja1,3 1. Stellenbosch Institute for Advanced Study, Wallenberg Research Centre at Stellenbosch University, Stellenbosch 7600, South Africa; 2. Department of Animal and Plant Sciences, The University of Sheffield, Sheffield S10 2TN, United Kingdom; and Department of Marine Sciences, University of Gothenburg, Tjärnö SE-45296 Strömstad, Sweden; 3. Institut des Sciences de l’Evolution, Unité Mixte de Recherche 5554 (Centre National de la Recherche Scientifique–Institut de Recherche pour le Développement–École pratique des hautes études), Université de Montpellier, 34095 Montpellier, France Submitted March 15, 2017; Accepted August 28, 2017; Electronically published December 15, 2017 abstract: During the process of speciation, populations may di- Introduction verge for traits and at their underlying loci that contribute barriers Understanding how reproductive isolation evolves is key fl to gene ow. -

Clinal Variation at Microsatellite Loci Reveals Historical Secondary Intergradation Between Glacial Races of Coregonus Artedi (Teleostei: Coregoninae)
Evolution, 55(11), 2001, pp. 2274±2286 CLINAL VARIATION AT MICROSATELLITE LOCI REVEALS HISTORICAL SECONDARY INTERGRADATION BETWEEN GLACIAL RACES OF COREGONUS ARTEDI (TELEOSTEI: COREGONINAE) JULIE TURGEON1,2,3 AND LOUIS BERNATCHEZ1,4 1Groupe Interuniversitaire de Recherche en OceÂanographie du QueÂbec, DeÂpartement de biologie, Universite Laval, Ste-Foy, QueÂbec G1K 7P4, Canada 2E-mail: [email protected] 4E-mail: [email protected] Abstract. Classical models of the spatial structure of population genetics rely on the assumption of migration-drift equilibrium, which is seldom met in natural populations having only recently colonized their current range (e.g., postglacial). Population structure then depicts historical events, and counfounding effects due to recent secondary contact between recently differentiated lineages can further counfound analyses of association between geographic and genetic distances. Mitochondrial polymorphisms have revealed the existence of two closely related lineages of the lake cisco, Coregonus artedi, whose signi®cantly different but overlaping geographical distributions provided a weak signal of past range fragmentation blurred by putative subsequent extensive secondary contacts. In this study, we analyzed geographical patterns of genetic variation at seven microsatellite loci among 22 populations of lake cisco located along the axis of an area covered by proglacial lakes 12,000±8000 years ago in North America. The results clearly con®rmed the existence of two genetically distinct races characterized by different sets of microsatellite alleles whose frequencies varied clinally across some 3000 km. Equilibrium and nonequilibrium analyses of isolation by distance revealed historical signal of gene ¯ow resulting from the nearly complete admixture of these races following neutral secondary contacts in their historical habitat and indicated that the colonization process occurred by a stepwise expansion of an eastern (Atlantic) race into a previously established Mississippian race. -

Plant Speciation
PLANT SPECIATION Niarsi Merry Hemelda, M.Si. MK Keanekaragaman Tumbuhan Departemen Biologi FMIPA -UI OUTLINE: Evolution Modes of plant speciation Features of plant evolution Speciation EVOLUTION SPECIES The cumulative The basic biological change in the heritable unit around which characteristics of a classifications are population over time. based. Speciation: an evolutionary process by which a new species comes into being. Evolution Microevolution Macroevolution • Microevolution is a change in generally refers to evolution gene frequency in a population in above the species level. short period of time. • Processes that can directly affect gene frequencies in a population: (mutation, migration, genetic drift, non random mating, natural selection) Patterns of evolution: A. Divergent Evolution: the two species gradually become increasingly different. B. Convergent Evolution: species of different ancestry begin to share analogous traits because of a shared environment or other selection pressure C. Parallel Evolution: two species evolve independently of each other, maintaining the same level of similarity. Parallel evolution usually occurs between unrelated species that do not occupy the same or similar niches in a given habitat. How a new species originate: • Species are created by a series of evolutionary processes. • Classically, speciation has been viewed as a three stage process: oIsolation of populations. oDivergence in traits of separated populations (e.g. mating system or habitat use). oReproductive isolation of populations that maintains -
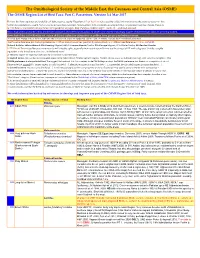
OSME List V3.4 Passerines-2
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa: Part C, Passerines. Version 3.4 Mar 2017 For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates either added information since the previous version or that further documentation is sought. Not all synonyms have been examined. Serial numbers (SN) are merely an administrative conveninence and may change. Please do not cite them as row numbers in any formal correspondence or papers. Key: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. The Passerine Reference List (including References for Hypothetical passerines [see Part E] and explanations of Abbreviated References) follows at Part D. Notes↓ & Status abbreviations→ BM=Breeding Migrant, SB/SV=Summer Breeder/Visitor, PM=Passage Migrant, WV=Winter Visitor, RB=Resident Breeder 1. PT=Parent Taxon (used because many records will antedate splits, especially from recent research) – we use the concept of PT with a degree of latitude, roughly equivalent to the formal term sensu lato , ‘in the broad sense’. 2. The term 'report' or ‘reported’ indicates the occurrence is unconfirmed. -

Chv), Coastal Clay (Cc), and Plateau Harsh Voice (Phv
Heredity (1978),40 (3), 339-348 INTRA-SPECIFIC DIVERGENCE IN THE WESTERN AUSTRALIAN FROG RANIDELLA INSIGNIFERA I. THE EVIDENCE FROM GENE FREQUENCIES AND GENETIC DISTANCE JENEFER N. BLACKWELL* Department of Zoology, University of Western Australia, Nedlands, Western Australia 6009 Received26.viii.77 SUMMARY Analysis of gene frequencies at ten enzyme loci (six polymorphic) failed to produce any evidence that races exist in the frog Ranidellainsign/fera associated with the edaphic feature of sand and clay based ponds. Most polymorphic loci exhibited macrogeographic uniformity of allele frequencies but in no case had any one allele become fixed in any one population. Nor were there any alleles unique to sand populations only or to clay populations only. Genetic distance algorithms failed to show any intra-specific divergence intermediate between inter-population and inter-sibling species levels. 1. INTRODUCTION ON the basis of aurally distinguishable differences in male mating call Main (1957) divided the leptodactylid frog species, Ranidella insignfera (recently removed from the genus Crinia by Blake, 1973) from south-western Australia into three call-types: Coastal harsh voice (Chv), Coastal clay (Cc), and Plateau harsh voice (Phv). Phv appeared to remain unchanged over its range and absence of intergrading calls with either Chv or Cc indicated a lack of gene flow. Phv was therefore regarded as a valid species, R. pseud- insignèra, reproductively isolated from R. insignjfera. Intergradation of calls, in some localities abruptly and in others in a more gradual manner, demon- strated the presence of gene flow between Chv and Cc which were then regarded as two races of one polytypic species, R. -

Analysis of Host-Parasite Cospeciation: Effects of Spatial and Temporal Scale
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1996 Analysis of Host-Parasite Cospeciation: Effects of Spatial and Temporal Scale. James W. Demastes Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Demastes, James W., "Analysis of Host-Parasite Cospeciation: Effects of Spatial and Temporal Scale." (1996). LSU Historical Dissertations and Theses. 6331. https://digitalcommons.lsu.edu/gradschool_disstheses/6331 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type o f computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -

Mayr, Dobzhansky, and Bush and the Complexities of Sympatric Speciation in Rhagoletis
Colloquium Mayr, Dobzhansky, and Bush and the complexities of sympatric speciation in Rhagoletis Jeffrey L. Feder†‡, Xianfa Xie†, Juan Rull§, Sebastian Velez†, Andrew Forbes†, Brian Leung†¶, Hattie Dambroski†ʈ, Kenneth E. Filchak†, and Martin Aluja§ †Department of Biological Sciences, University of Notre Dame, Galvin Life Science Center, P.O. Box 369, Notre Dame, IN 46556-0369; §Instituto de Ecologı´a, Asociacio´n Civil, Kilometro 2.5 Antigua Carretera a Coatepec 351, 91070 Xalapa, Veracruz, Mexico; ¶Department of Biology, McGill School of Environment, McGill University, Montreal, QB, Canada H3A 1B1; and ʈCereal Disease Laboratory, United States Department of Agriculture, Agricultural Research Service, Midwest Area Office, University of Minnesota, 1551 Lindig Avenue, St. Paul, MN 55104 The Rhagoletis pomonella sibling species complex is a model for plants. Theodosius Dobzhansky (5, 6) pioneered the genetic sympatric speciation by means of host plant shifting. However, study of speciation, mapping genetic factors responsible for genetic variation aiding the sympatric radiation of the group in the hybrid sterility and inviability and surveying natural populations United States may have geographic roots. Inversions on chromo- to assess levels of genetic variation. He crystallized the view that somes 1–3 affecting diapause traits adapting flies to differences in speciation represents the transformation of within-population host fruiting phenology appear to exist in the United States variation into between-taxa differences through the evolution of because of a series of secondary introgression events from Mexico. inherent reproductive isolating barriers. Dobzhansky (6) also Here, we investigate whether these inverted regions of the ge- was a strong advocate of genetic coadaptation, especially with nome may have subsequently evolved to become more recalcitrant regard to balanced polymorphisms in the form of chromosomal to introgression relative to collinear regions, consistent with new inversions. -

Barrington, D.S., C.H. Haufler, and C.R. Werth. 1989. Hybridization
AmericanFern Journal 79(2): 55-64 (1989) Hybridization, Reticulation, and Species Concepts in the Ferns DAVID S. BARRINGTON Department of Botany, University of Vermont, Burlington, Vermont 05405-0086 CHRISTOPHER H. HAUFLER Department of Botany, University of Kansas, Lawrence, Kansas 66045 CHARLES R. WERTH Department of Biological Sciences, Texas Tech University, Lubbock, Texas 79409 Hybrids and hybrid species are common among ferns, and they account for many of the problems in species definition in the group.Most systematic inquiry into the evolutionary process in ferns has addressed hybrid species, because meaningful explanations of their origins are feasible (Manton,1950). As a result, complexes of hybrids, hybrid species, and their progenitor species have been popular subjects for experimental work. Here, we address the definition and changing perception of these hybrid species in the light of improvements in the data available to systematists. Once we have established basic definitions, we demonstrate the utility of recent advances in defining hybrid species of ferns. With this orientation, we investigate the status of hybrid species in the context of reigning species concepts. Renewed reproductive interactionbetween populations or species following a period of isolation characterizesall hybrids;hence hybrids are often spoken of as the products of secondary contact. Hybrids are unique in that they arise when isolating mechanisms fail; thus they are evolutionarily a consequence of the disruption of the divergence process that leads to ordinary (primary)species. Consequently, the hybrid is at once a novelty and a rehash: it is a novel combination of genetic and morphological features already present in its progenitors. These features need not be intermediate: see Grant (1975) on transgressive segregation and Barrington, 1986a. -

Coevolution and Cospeciation in a Bark-Beetle Fungal Symbiosis
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2015 Coevolution and cospeciation in a bark-beetle fungal symbiosis Ryan Russell Bracewell Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Bracewell, Ryan Russell, "Coevolution and cospeciation in a bark-beetle fungal symbiosis" (2015). Graduate Student Theses, Dissertations, & Professional Papers. 10784. https://scholarworks.umt.edu/etd/10784 This Dissertation is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. COEVOLUTION AND COSPECIATION IN A BARK-BEETLE FUNGAL SYMBIOSIS By RYAN RUSSELL BRACEWELL B.S. Bioagricultural Sciences and Pest Management, Colorado State University, Fort Collins, CO, 2002 M.S. Ecology, Utah State University, Logan, UT, 2009 Dissertation presented in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Forestry and Conservation Sciences The University of Montana Missoula, MT December 2015 Approved by: Sandy Ross, Dean of The Graduate School Graduate School Dr. Diana Six, Chair Department of Ecosystem and Conservation Sciences Dr. Jeffrey Good Division of Biological Sciences Dr. John McCutcheon Division of Biological Sciences Dr. Michael Schwartz USDA Forest Service/College of Forestry and Conservation Dr. Ylva Lekberg MPG Ranch/College of Forestry and Conservation ProQuest Number: 10096854 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted.