Light Scattering Studies of Amphiphilic Drugs Promethazine Hydrochloride and Imipramine Hydrochloride in Aqueous Electrolyte Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thioridazine Induces Apoptosis of Multidrug-Resistant Mouse Lymphoma Cells Transfected with the Human ABCB1 and Inhibits the Expression of P-Glycoprotein
ANTICANCER RESEARCH 31: 4201-4206 (2011) Thioridazine Induces Apoptosis of Multidrug-resistant Mouse Lymphoma Cells Transfected with the Human ABCB1 and Inhibits the Expression of P-Glycoprotein GABRIELLA SPENGLER1,3, JOSEPH MOLNAR1, MIGUEL VIVEIROS2,4 and LEONARD AMARAL2,3,4 1Institute of Medical Microbiology and Immunobiology, Faculty of Medicine, University of Szeged, Szeged, Hungary; 2Group of Mycobacteriology, Unit of Microbiology and 3Unit of Parasitology and Medical Microbiology (UPMM), Institute of Hygiene and Tropical Medicine, Universidade Nova de Lisboa, Lisboa, Portugal; 4Cost Action BM0701 (ATENS) of the European Commission, Brussels, Belgium Abstract. Aim: Chlorpromazine has activity against a large therapeutic aspects, and low availability of effective but also variety of cancer types. However, this phenothiazine produces costly forms of therapy (chemotherapy, radiotherapy). a plethora of serious side-effects. We have studied thioridazine Regardless of economic support, chemotherapy of cancer is (TZ), a phenothiazine neuroleptic that is much milder, for highly problematic, especially when therapy promotes the activity against multidrug-resistant (MDR) cancer cells, as development of multidrug resistance (MDR), hence cancer well as against the overexpressed ABCB1 transporter (P- becomes refractory not only to the initial chemotherapeutic glycoprotein) that is the cause for the MDR phenotype of these agent, but to many other anticancer drugs as well (1, 2). The cancer cells. Materials and Methods: MDR mouse T- development of an MDR phenotype of cancer cell has been lymphoma cells, transfected with the human gene ABCB1 that known for many decades to be due to the overexpression of codes for the transporter ABCB1, were incubated with TZ for transporters that extrude the anticancer agent before it various periods of time and examined for evidence of reaches its intended target (3). -

FDA Approved Drugs with Broad Anti-Coronaviral Activity Inhibit SARS-Cov-2 in Vitro
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.25.008482; this version posted March 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. FDA approved drugs with broad anti-coronaviral activity inhibit SARS-CoV-2 in vitro Stuart Weston1, Rob Haupt1, James Logue1, Krystal Matthews1 and Matthew B. Frieman*1 1 - Department of Microbiology and Immunology, University of Maryland School of Medicine, 685 W. Baltimore St., Room 380, Baltimore, MD, 21201, USA *Corresponding author. Email: [email protected] Key words: SARS-CoV-2, nCoV-2019, COVID-19, drug repurposing, FDA approved drugs, antiviral therapeutics, pandemic, chloroquine, hydroxychloroquine AbstraCt SARS-CoV-2 emerged in China at the end of 2019 and has rapidly become a pandemic with over 400,000 recorded COVID-19 cases and greater than 19,000 recorded deaths by March 24th, 2020 (www.WHO.org) (1). There are no FDA approved antivirals or vaccines for any coronavirus, including SARS-CoV-2 (2). Current treatments for COVID-19 are limited to supportive therapies and off-label use of FDA approved drugs (3). Rapid development and human testing of potential antivirals is greatly needed. A potentially quicker way to test compounds with antiviral activity is through drug re-purposing (2, 4). Numerous drugs are already approved for use in humans and subsequently there is a good understanding of their safety profiles and potential side effects, making them easier to test in COVID-19 patients. -

NORPRAMIN® (Desipramine Hydrochloride Tablets USP)
NORPRAMIN® (desipramine hydrochloride tablets USP) Suicidality and Antidepressant Drugs Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of NORPRAMIN or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. NORPRAMIN is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use.) DESCRIPTION NORPRAMIN® (desipramine hydrochloride USP) is an antidepressant drug of the tricyclic type, and is chemically: 5H-Dibenz[bƒ]azepine-5-propanamine,10,11-dihydro-N-methyl-, monohydrochloride. 1 Reference ID: 3536021 Inactive Ingredients The following inactive ingredients are contained in all dosage strengths: acacia, calcium carbonate, corn starch, D&C Red No. 30 and D&C Yellow No. 10 (except 10 mg and 150 mg), FD&C Blue No. 1 (except 25 mg, 75 mg, and 100 mg), hydrogenated soy oil, iron oxide, light mineral oil, magnesium stearate, mannitol, polyethylene glycol 8000, pregelatinized corn starch, sodium benzoate (except 150 mg), sucrose, talc, titanium dioxide, and other ingredients. -

A Drug Repositioning Approach Identifies Tricyclic Antidepressants As Inhibitors of Small Cell Lung Cancer and Other Neuroendocrine Tumors
Published OnlineFirst September 26, 2013; DOI: 10.1158/2159-8290.CD-13-0183 RESEARCH ARTICLE A Drug Repositioning Approach Identifi es Tricyclic Antidepressants as Inhibitors of Small Cell Lung Cancer and Other Neuroendocrine Tumors Nadine S. Jahchan 1 , 2 , Joel T. Dudley 1 , Pawel K. Mazur 1 , 2 , Natasha Flores 1 , 2 , Dian Yang 1 , 2 , Alec Palmerton 1 , 2 , Anne-Flore Zmoos 1 , 2 , Dedeepya Vaka 1 , 2 , Kim Q.T. Tran 1 , 2 , Margaret Zhou 1 , 2 , Karolina Krasinska 3 , Jonathan W. Riess 4 , Joel W. Neal 5 , Purvesh Khatri 1 , 2 , Kwon S. Park 1 , 2 , Atul J. Butte 1 , 2 , and Julien Sage 1 , 2 Downloaded from cancerdiscovery.aacrjournals.org on September 29, 2021. © 2013 American Association for Cancer Research. Published OnlineFirst September 26, 2013; DOI: 10.1158/2159-8290.CD-13-0183 ABSTRACT Small cell lung cancer (SCLC) is an aggressive neuroendocrine subtype of lung cancer with high mortality. We used a systematic drug repositioning bioinformat- ics approach querying a large compendium of gene expression profi les to identify candidate U.S. Food and Drug Administration (FDA)–approved drugs to treat SCLC. We found that tricyclic antidepressants and related molecules potently induce apoptosis in both chemonaïve and chemoresistant SCLC cells in culture, in mouse and human SCLC tumors transplanted into immunocompromised mice, and in endog- enous tumors from a mouse model for human SCLC. The candidate drugs activate stress pathways and induce cell death in SCLC cells, at least in part by disrupting autocrine survival signals involving neurotransmitters and their G protein–coupled receptors. The candidate drugs inhibit the growth of other neuroendocrine tumors, including pancreatic neuroendocrine tumors and Merkel cell carcinoma. -

Male Anorgasmia: from “No” to “Go!”
Male Anorgasmia: From “No” to “Go!” Alexander W. Pastuszak, MD, PhD Assistant Professor Center for Reproductive Medicine Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Disclosures • Endo – speaker, consultant, advisor • Boston Scientific / AMS – consultant • Woven Health – founder, CMO Objectives • Understand what delayed ejaculation (DE) and anorgasmia are • Review the anatomy and physiology relevant to these conditions • Review what is known about the causes of DE and anorgasmia • Discuss management of DE and anorgasmia Definitions Delayed Ejaculation (DE) / Anorgasmia • The persistent or recurrent delay, difficulty, or absence of orgasm after sufficient sexual stimulation that causes personal distress Intravaginal Ejaculatory Latency Time (IELT) • Normal (median) à 5.4 minutes (0.55-44.1 minutes) • DE à mean IELT + 2 SD = 25 minutes • Incidence à 2-11% • Depends in part on definition used J Sex Med. 2005; 2: 492. Int J Impot Res. 2012; 24: 131. Ejaculation • Separate event from erection! • Thus, can occur in the ABSENCE of erection! Periurethral muscle Sensory input - glans (S2-4) contraction Emission Vas deferens contraction Sympathetic input (T12-L1) SV, prostate contraction Bladder neck contraction Expulsion Bulbocavernosus / Somatic input (S1-3) spongiosus contraction Projectile ejaculation J Sex Med. 2011; 8 (Suppl 4): 310. Neurochemistry Sexual Response Areas of the Brain • Pons • Nucleus paragigantocellularis Neurochemicals • Norepinephrine, serotonin: • Inhibit libido, -

HALDOL Decanoate 50 (Haloperidol)
HALDOL® Decanoate 50 (haloperidol) HALDOL® Decanoate 100 (haloperidol) For IM Injection Only WARNING Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. HALDOL Decanoate is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS). DESCRIPTION Haloperidol decanoate is the decanoate ester of the butyrophenone, HALDOL (haloperidol). It has a markedly extended duration of effect. It is available in sesame oil in sterile form for intramuscular (IM) injection. The structural formula of haloperidol decanoate, 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]-4 piperidinyl decanoate, is: Haloperidol decanoate is almost insoluble in water (0.01 mg/mL), but is soluble in most organic solvents. -
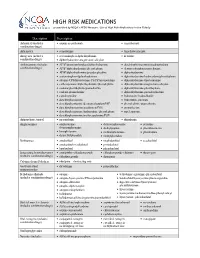
HIGH RISK MEDICATIONS As Specified by NCQA’S HEDIS Measure: Use of High Risk Medications in the Elderly
HIGH RISK MEDICATIONS as specified by NCQA’s HEDIS Measure: Use of High Risk Medications in the Elderly Description Prescription Antianxiety (includes • aspirin-meprobamate • meprobamate combination drugs) Antiemetics • scopolamine • trimethobenzamide Analgesics (includes • acetaminophen-diphenhydramine • ketorolac combination drugs) • diphenhydramine-magnesium salicylate Antihistamines (includes • APAP/dextromethorphan/diphenhydramine • dexchlorpheniramine-pseudoephedrine combination drugs) • APAP/diphenhydramine/phenylephrine • dextromethorphan-promethazine • APAP/diphenhydramine/pseudoephedrine • diphenhydramine • acetaminophen-diphenhydramine • diphenhydramine/hydrocodone/phenylephrine • atropine/CPM/hyoscyamine/PE/PPA/scopolamine • diphenhydramine-tripelennamine • carbetapentane/diphenhydramine/phenylephrine • diphenhydramine-magnesium salicylate • codeine/phenylephrine/promethazine • diphenhydramine-phenylephrine • codeine-promethazine • diphenhydramine-pseudoephedrine • cyproheptadine • hydroxyzine hydrochloride • dexchlorpheniramine • hydroxyzine pamoate • dexchlorpheniramine/dextromethorphan/PSE • phenylephrine-promethazine • dexchlorpheniramine/guaifenesin/PSE • promethazine • dexchlorpheniramine/hydrocodone/phenylephrine • tripelennamine • dexchlorpheniramine/methscopolamine/PSE Antipsychotic, typical • mesoridazine • thioridazine Amphetamines • amphetamine- • dextroamphetamine • pemoline dextroamphetamine • diethylpropion • phendimetrazine • benzphetamine • methamphetamine • phentermine • dexmethylphenidate • methylphenidate -

Determination of Thiazinamium, Promazine and Promethazine in Pharmaceutical Formulations Using a CZE Method Francisco J
Analytica Chimica Acta 535 (2005) 101–108 Determination of thiazinamium, promazine and promethazine in pharmaceutical formulations using a CZE method Francisco J. Lara, Ana M. Garc´ıa-Campana˜ ∗, Ferm´ın Ales-Barrero,´ Juan M. Bosque-Sendra Department of Analytical Chemistry, Faculty of Sciences, University of Granada, Avd. Fuente Nueva s/n, E-18071 Granada, Spain Received 26 July 2004; received in revised form 30 November 2004; accepted 30 November 2004 Available online 15 January 2005 Abstract We present the validation of a developed method using capillary zone electrophoresis (CZE) for quantitative analysis of three phenoth- iazines: thiazinamium methylsulphate (TMS), promazine hydrochloride (PMH) and promethazine hydrochloride (PTH) in pharmaceutical formulations. The influence of various parameters affecting the CZE separation (buffer pH and concentration, acetonitrile percentage, capil- lary temperature and applied voltage) was investigated by means of multivariate optimization using experimental designs so as to obtain the highest efficiency. The method allows the separation of the phenothiazines in 5.0 min at optimized conditions: 100 mM tris(hydroxymethyl)- aminomethane (Tris) buffer at a pH 8.0, 15% acetonitrile, capillary with 58.5 cm in length at 25 ◦C and a voltage of 30 kV.Detection occurred at 254 nm. Acceptable precision (relative standard deviation (R.S.D.) 5.3%) and linearity were achieved using the internal standard (IS) method. The limits of detection (LODs) were 2.8 gml−1 for TMS and 3.3 gml−1 for PMH and PTH. The method has been successfully applied in the analysis of pharmaceutical formulations. © 2004 Elsevier B.V. All rights reserved. Keywords: Capillary zone electrophoresis; Thiazinamium methylsulphate; Promazine; Promethazine; Pharmaceutical analysis 1. -

Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gutlbrain Interaction): a Rome Foundation Working Team Report Douglas A
Gastroenterology 2018;154:1140–1171 SPECIAL REPORT Neuromodulators for Functional Gastrointestinal Disorders (Disorders of GutLBrain Interaction): A Rome Foundation Working Team Report Douglas A. Drossman,1,2 Jan Tack,3 Alexander C. Ford,4,5 Eva Szigethy,6 Hans Törnblom,7 and Lukas Van Oudenhove8 1Center for Functional Gastrointestinal and Motility Disorders, University of North Carolina, Chapel Hill, North Carolina; 2Center for Education and Practice of Biopsychosocial Care and Drossman Gastroenterology, Chapel Hill, North Carolina; 3Translational Research Center for Gastrointestinal Disorders, University of Leuven, Leuven, Belgium; 4Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, United Kingdom; 5Leeds Gastroenterology Institute, St James’s University Hospital, Leeds, United Kingdom; 6Departments of Psychiatry and Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania; 7Departments of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; and 8Laboratory for BrainÀGut Axis Studies, Translational Research Center for Gastrointestinal Disorders, University of Leuven, Leuven, Belgium BACKGROUND & AIMS: Central neuromodulators (antide- summary information and guidelines for the use of central pressants, antipsychotics, and other central nervous neuromodulators in the treatment of chronic gastrointestinal systemÀtargeted medications) are increasingly used for treat- symptoms and FGIDs. Further studies are needed to confirm ment -

Promethazine-Chlorpromazine Combination in the Treatment of Unmanageable Psychotic Patients Armando R
Henry Ford Hospital Medical Journal Volume 17 | Number 4 Article 10 12-1969 Promethazine-Chlorpromazine Combination in the Treatment of Unmanageable Psychotic Patients Armando R. Favazza Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Chemicals and Drugs Commons, Life Sciences Commons, Medical Specialties Commons, Psychiatry and Psychology Commons, and the Public Health Commons Recommended Citation Favazza, Armando R. (1969) "Promethazine-Chlorpromazine Combination in the Treatment of Unmanageable Psychotic Patients," Henry Ford Hospital Medical Journal : Vol. 17 : No. 4 , 305-310. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol17/iss4/10 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp. Med. Journal VoL 17, No. 4, 1969 Promethazine-chlorpromazine Combination in the Treatment of Unmanageable Psychotic Patients Armando R. Favazza, M.D.* Administering a combination of promethazine and chlorpromazine to patients with a "galloping psychosis" has an antipsychotic and tranquilizing effect which calms them down to a more manageable and less aggressive state. The drugs are chemically similar; promethazine's actions are strongly potentiated in the combina tion, so large doses must be given under careful supervision. Case histories demonstrate successful short term management of acutely psychotic, aggressive patients who were a danger to themselves and others. Acute, intense psychotic episodes common in the author's experience for can pose difficult problems for both some patients to receive massive doses the patient and those who care for him. -

Medications to Be Avoided Or Used with Caution in Parkinson's Disease
Medications To Be Avoided Or Used With Caution in Parkinson’s Disease This medication list is not intended to be complete and additional brand names may be found for each medication. Every patient is different and you may need to take one of these medications despite caution against it. Please discuss your particular situation with your physician and do not stop any medication that you are currently taking without first seeking advice from your physician. Most medications should be tapered off and not stopped suddenly. Although you may not be taking these medications at home, one of these medications may be introduced while hospitalized. If a hospitalization is planned, please have your neurologist contact your treating physician in the hospital to advise which medications should be avoided. Medications to be avoided or used with caution in combination with Selegiline HCL (Eldepryl®, Deprenyl®, Zelapar®), Rasagiline (Azilect®) and Safinamide (Xadago®) Medication Type Medication Name Brand Name Narcotics/Analgesics Meperidine Demerol® Tramadol Ultram® Methadone Dolophine® Propoxyphene Darvon® Antidepressants St. John’s Wort Several Brands Muscle Relaxants Cyclobenzaprine Flexeril® Cough Suppressants Dextromethorphan Robitussin® products, other brands — found as an ingredient in various cough and cold medications Decongestants/Stimulants Pseudoephedrine Sudafed® products, other Phenylephrine brands — found as an ingredient Ephedrine in various cold and allergy medications Other medications Linezolid (antibiotic) Zyvox® that inhibit Monoamine oxidase Phenelzine Nardil® Tranylcypromine Parnate® Isocarboxazid Marplan® Note: Additional medications are cautioned against in people taking Monoamine oxidase inhibitors (MAOI), including other opioids (beyond what is mentioned in the chart above), most classes of antidepressants and other stimulants (beyond what is mentioned in the chart above). -

SELECTIVE SEROTONIN REUPTAKE INHIBITORS SEXUAL FUNCTION K. Demyttenaere0 and D. Vanderschueren" University Hospital Gasthui
* 1995 Elsevier Science B. V. All rights reserved. The Pharmacology of Sexual Function and Dysfunction J. Bancroft, editor 327 SELECTIVE SEROTONIN REUPTAKE INHIBITORS AND SEXUAL FUNCTION K. Demyttenaere0 and D. Vanderschueren" University Hospital Gasthuisberg (K.D. Leuven) ° Department of Psychiatry and Institute for Family and Sexological Sciences " Department of Endocrinology - Andrology Unit Herestraat 49 - B 3000 Leuven, Belgium dedicated to dr. Hugo De Cuyper (18.1.1946 - 13.9.1994) Introduction Selective Serotonin Reuptake Inhibitors (SSRIs : fluvoxamine -Floxyfral® or Fevarin®-, fluoxetine -Prozac®-, sertraline - Serlain® or Zoloft®-, paroxetine -Seroxat® or Paxil®- and citalopram -Cipramil®-) are potent and competitive inhibitors of the high affinity neuronal re-uptake mechanism for serotonin. These drugs also inhibit the re-uptake of noradrenaline and, generally to a lesser extent, dopamine, at higher concentrations. Paroxetine is the most potent serotonin uptake inhibitor in vitro of the drugs investigated, and citalopram is the most selective inhibitor of serotonin re-uptake, with paroxetine being more selective than fluoxetine, fluvoxamine and sertraline. Of the drugs investigated, only sertraline is a more potent inhibitor of dopamine compared with noradrenaline uptake. SSRIs are not only as effective as the tricyclic antidepressants in the treatment of major depression but are also effective in the treatment of obsessive-compulsive disorder, panic disorder, eating disorders and premenstrual syndrome (1). Although the latency of onset to therapeutic response seems to be somewhat longer, most psychiatrists consider SSRIs the initital drug of choice in the treatment of depressive disorders. Indeed, these drugs are usually better tolerated since they do not produce muscarinic, histaminic and alpha adrenergic side effects.