Reading Chest Radiographs in the Critically Ill (Part II): Radiography of Lung Pathologies Common in the ICU Patient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diagnosing Pulmonary Embolism M Riedel
309 Postgrad Med J: first published as 10.1136/pgmj.2003.007955 on 10 June 2004. Downloaded from REVIEW Diagnosing pulmonary embolism M Riedel ............................................................................................................................... Postgrad Med J 2004;80:309–319. doi: 10.1136/pgmj.2003.007955 Objective testing for pulmonary embolism is necessary, embolism have a low long term risk of subse- quent VTE.2 5–7 because clinical assessment alone is unreliable and the consequences of misdiagnosis are serious. No single test RISK FACTORS AND RISK has ideal properties (100% sensitivity and specificity, no STRATIFICATION risk, low cost). Pulmonary angiography is regarded as the The factors predisposing to VTE broadly fit Virchow’s triad of venous stasis, injury to the final arbiter but is ill suited for diagnosing a disease vein wall, and enhanced coagulability of the present in only a third of patients in whom it is suspected. blood (box 1). The identification of risk factors Some tests are good for confirmation and some for aids clinical diagnosis of VTE and guides decisions about repeat testing in borderline exclusion of embolism; others are able to do both but are cases. Primary ‘‘thrombophilic’’ abnormalities often non-diagnostic. For optimal efficiency, choice of the need to interact with acquired risk factors before initial test should be guided by clinical assessment of the thrombosis occurs; they are usually discovered after the thromboembolic event. Therefore, the likelihood of embolism and by patient characteristics that risk of VTE is best assessed by recognising the may influence test accuracy. Standardised clinical presence of known ‘‘clinical’’ risk factors. estimates can be used to give a pre-test probability to However, investigations for thrombophilic dis- orders at follow up should be considered in those assess, after appropriate objective testing, the post-test without another apparent explanation. -
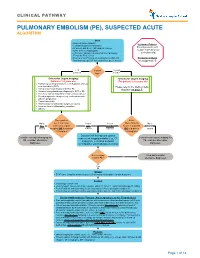
Pulmonary Embolism (Pe), Suspected Acute Algorithm
CLINICAL PATHWAY PULMONARY EMBOLISM (PE), SUSPECTED ACUTE ALGORITHM Start · Room air pulse oximetry Inclusion Criteria: · Consider supplemental oxygen · IV access and STAT CBC and DIC screen Patient presents with · Urine β-HCG, if appropriate suspected Pulmonary · CXR (PA + lateral), EKG and call the Cardiology Embolism (PE) Fellow for Echo review · Review criteria* for urgent imaging for possible PE Exclusion Criteria based on age-specific risk factors (see green boxes) No suspected PE < 18 Patient 18 years years old age? or older *Criteria for Urgent Imaging: *Criteria for Urgent Imaging: Patients < 18 years old For patients ≥ 18 years old · Painful leg swelling or known recent diagnosis of deep vein thrombosis (DVT) Please refer to the Wells Criteria · Family or personal history of DVT or PE Algorithm on page 7. · Known clotting disorder predisposing to DVT or PE · Recent or current indwelling central venous catheter · Elevated systemic estrogen (e.g., oral contraceptive pill use, pregnancy) · Recent immobility · Recent major or orthopedic surgery or trauma · Acute or chronic inflammatory condition · Obesity Does patient Is the No to meet 1 or more Yes to Yes to Wells Criteria No to ALL criteria for urgent ANY EITHER Score > 4 points BOTH criteria imaging OR is d-dimer criteria criterion OR is d-dimer criteria > 0.5ug/mL? > 0.5ug/mL? Discuss CXR findings and optimal Consider non-urgent imaging for subsequent imaging modality (e.g., CT Consider non-urgent imaging for PE, consider alternative angiogram, ventilation perfusion PE, consider -

CHEST RADIOLOGY: Goals and Objectives
Harlem Hospital Center Department of Radiology Residency Training Program CHEST RADIOLOGY: Goals and Objectives ROTATION 1 (Radiology Years 1): Resident responsibilities: • ED chest CTs • Inpatient and outpatient plain films including the portable intensive care unit radiographs • Consultations with referring clinicians MEDICAL KNOWLEDGE: • Residents must demonstrate knowledge about established and evolving biomedical, clinical, and cognitive sciences and the application of this knowledge to patient care. At the end of the rotation, the resident should be able to: • Identify normal radiographic and CT anatomy of the chest • Identify and describe common variants of normal, including aging changes. • Demonstrate a basic knowledge of radiographic interpretation of atelectasis, pulmonary infection, congestive heart failure, pleural effusion and common neoplastic diseases of the chest • Identify the common radiologic manifestation of thoracic trauma, including widened mediastinum, signs of aortic laceration, pulmonary contusion/laceration, esophageal and diaphragmatic rupture. • Know the expected postoperative appearance in patients s/p thoracic surgery and the expected location of the life support and monitoring devices on chest radiographs of critically ill patients (intensive care radiology); be able to recognize malpositioned devices. • Identify cardiac enlargement and know the radiographic appearance of the dilated right vs. left atria and right vs. left ventricles, and pulmonary vascular congestion • Recognize common life-threatening -

Chest Radiology: a Resident's Manual
Chest Radiology: A Resident's Manual Bearbeitet von Johannes Kirchner 1. Auflage 2011. Buch. 300 S. Hardcover ISBN 978 3 13 153871 0 Format (B x L): 23 x 31 cm Weitere Fachgebiete > Medizin > Sonstige Medizinische Fachgebiete > Radiologie, Bildgebende Verfahren Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. 1 Heart Failure Acute left heart failure is most commonly caused by a hyperten- " Compare pulmonary vessels that are equidistant to a central sive crisis. Radiographic signs on the plain chest radiograph ob- point in the respective hilum. tained with the patient standing include: " Compare the diameter of a random easily identifiable superior " Redistribution of pulmonary perfusion lobe artery (often the anterior segmental artery is most easily " Presence of interstitial patterns (Kerley lines, peribronchial identifiable) with the diameter of the corresponding ipsilateral cuffing) bronchus (Fig. 1.62). " Alveolar densities with indistinct vascular structures (ad- vanced stage) As the pulmonary artery and corresponding ipsilateral bronchus " Pleural effusions are normally of precisely equal diameter, a larger arterial diameter is indicative of redistribution of perfusion (Fig. 1.63). The diagnos- All of these signs are essentially attributable to increased fluid tic criteria of caudal-to-cranial redistribution cannot be evaluated content in the abnormally heavy “wet” lung. The fluid accumula- on radiographs obtained in the supine patient. -

Chapter 17 Dyspnea Sabina Braithwaite and Debra Perina
Chapter 17 Dyspnea Sabina Braithwaite and Debra Perina ■ PERSPECTIVE Pathophysiology Dyspnea is the term applied to the sensation of breathlessness The actual mechanisms responsible for dyspnea are unknown. and the patient’s reaction to that sensation. It is an uncomfort- Normal breathing is controlled both centrally by the respira- able awareness of breathing difficulties that in the extreme tory control center in the medulla oblongata, as well as periph- manifests as “air hunger.” Dyspnea is often ill defined by erally by chemoreceptors located near the carotid bodies, and patients, who may describe the feeling as shortness of breath, mechanoreceptors in the diaphragm and skeletal muscles.3 chest tightness, or difficulty breathing. Dyspnea results Any imbalance between these sites is perceived as dyspnea. from a variety of conditions, ranging from nonurgent to life- This imbalance generally results from ventilatory demand threatening. Neither the clinical severity nor the patient’s per- being greater than capacity.4 ception correlates well with the seriousness of underlying The perception and sensation of dyspnea are believed to pathology and may be affected by emotions, behavioral and occur by one or more of the following mechanisms: increased cultural influences, and external stimuli.1,2 work of breathing, such as the increased lung resistance or The following terms may be used in the assessment of the decreased compliance that occurs with asthma or chronic dyspneic patient: obstructive pulmonary disease (COPD), or increased respira- tory drive, such as results from severe hypoxemia, acidosis, or Tachypnea: A respiratory rate greater than normal. Normal rates centrally acting stimuli (toxins, central nervous system events). -

Mayo Clinic Medical Manual This Page Intentionally Left Blank Mayo Clinic Medical Manual
Mayo Clinic Medical Manual This page intentionally left blank Mayo Clinic Medical Manual Editors Guilherme H. M. Oliveira, M.D. Gillian C. Nesbitt, M.D. Joseph G. Murphy, M.D. MAYO CLINIC SCIENTIFIC PRESS TAYLOR & FRANCIS GROUP ISBN 0849390877 The triple-shield Mayo logo and the words MAYO, MAYO CLINIC, and MAYO CLINIC SCIENTIFIC PRESS are marks of Mayo Foundation for Medical Education and Research. ©2006 by Mayo Foundation for Medical Education and Research. All rights reserved. This book is protected by copyright. No part of it may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means—electronic, mechanical, photocopying, record- ing, or otherwise—without the prior written consent of the copyright holder, except for brief quotations embodied in critical articles and reviews. Inquiries should be addressed to Scientific Publications, Plummer 10, Mayo Clinic, 200 First Street SW, Rochester, MN 55905. For order inquiries, contact Taylor & Francis Group, 6000 Broken Sound Parkway NW, Suite #300, Boca Raton, FL 33487. www.taylorandfrancis.com Catalog record is available from the Library of Congress. Care has been taken to confirm the accuracy of the information presented and to describe generally accepted practices. However, the authors, editors, and publisher are not responsible for errors or omissions or for any consequences from application of the information in this book and make no warranty, express or implied, with respect to the contents of the publication. This book should not be relied on apart from the advice of a qualified health care provider. The authors, editors, and publisher have exerted efforts to ensure that drug selection and dosage set forth in this text are in accordance with current recommendations and practice at the time of publication. -

Pleural Effusion, Hypovascularity in Lung Zone (Westermark’S Sign) & Pyramid Shape Infiltrate with Peak Directed to Hilus (Hampton’S Hump)
Author(s): Michele M. Nypaver, MD, 2011 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/ We have reviewed this material in accordance with U.S. Copyright Law and have tried to maximize your ability to use, share, and adapt it. Copyright holders of content included in this material should contact [email protected] with any questions, corrections, or clarification regarding the use of content. For more information about how to cite these materials visit http://open.umich.edu/privacy-and-terms-use. Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have questions about your medical condition. Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers. Citation Key for more information see: http://open.umich.edu/wiki/CitationPolicy Use + Share + Adapt { Content the copyright holder, author, or law permits you to use, share and adapt. } Public Domain – Government: Works that are produced by the U.S. Government. (17 USC § 105) Public Domain – Expired: Works that are no longer protected due to an expired copyright term. Public Domain – Self Dedicated: Works that a copyright holder has dedicated to the public domain. Creative Commons – Zero Waiver Creative Commons – Attribution License Creative Commons – Attribution Share Alike License Creative Commons – Attribution Noncommercial License Creative Commons – Attribution Noncommercial Share Alike License GNU – Free Documentation License Make Your Own Assessment { Content Open.Michigan believes can be used, shared, and adapted because it is ineligible for copyright. -

Pneumonia (CAP)
肺實質化病變與肺塌陷 胸腔內科周百謙醫師 Dr. Pai-chien Chou MD PhD Department of Thoracic Medicine Taipei Medical University Hospital Chest X-ray • P-A view • Lateral view • Oblique view • Lordotic view • Expiratory film • Decubitus view • Overpenetrated grid film The Elements of a chest x-ray (CXR) • The Broncho-vascular markings in the lung • The borders of the heart • The contours of the mediastinum and pleural space • The ribs and spine Segmental anatomy Segmental Anatomy Cardiomediastinal outlines on Chest X-ray Density of image ◆ Gas ◆ Water ◆ Fat ◆ Metal and bone ◆ Thinking of pathogenesis Basic thinking of a lesion on Chest X-ray ◆ Size ◆ Location (Silhouette sign) – Anterior, posterior – Which lobe is involved ◆ Intrapulmonary (Air bronchogram sign) ◆ Extrapulmonary (Incomplete border sign) Infiltrate in the lungs • Fluid accumulates in lung, predominate in the alveolar (airspace) compartment or the interstitial compartment. interstitial compartment Lymphatic compartment Alveolar unit Vascular unit Air space opacification The opacification is caused by fluid or solid material within the airways that causes a difference in the relative attenuation of the lung: • transudate, e.g. pulmonary edema secondary to heart failure • pus, e.g. bacterial pneumonia • blood, e.g. pulmonary hemorrhage • cells, e.g. bronchoalveolar carcinoma • protein, e.g. alveolar proteinosis • fat, e.g. lipoid pneumonia • gastric contents, e.g. aspiration pneumonia • water, e.g. drowning When considering the likely causes of airspace opacification, it is useful to determine chronicity -

Signs in Chest Imaging
Diagn Interv Radiol 2011; 17:18–29 CHEST IMAGING © Turkish Society of Radiology 2011 PICTORIAL ESSAY Signs in chest imaging Oktay Algın, Gökhan Gökalp, Uğur Topal ABSTRACT adiological practice includes classification of illnesses with similar A radiological sign can sometimes resemble a particular object characteristics through recognizable signs. Knowledge of and abil- or pattern and is often highly suggestive of a group of similar pathologies. Awareness of such similarities can shorten the dif- R ity to recognize these signs can aid the physician in shortening ferential diagnosis list. Many such signs have been described the differential diagnosis list and deciding on the ultimate diagnosis for for X-ray and computed tomography (CT) images. In this ar- ticle, we present the most frequently encountered plain film a patient. In this report, 23 important and frequently seen radiological and CT signs in chest imaging. These signs include for plain signs are presented and described using chest X-rays, computed tomog- films the air bronchogram sign, silhouette sign, deep sulcus raphy (CT) images, illustrations and photographs. sign, Continuous diaphragm sign, air crescent (“meniscus”) sign, Golden S sign, cervicothoracic sign, Luftsichel sign, scim- itar sign, doughnut sign, Hampton hump sign, Westermark Plain films sign, and juxtaphrenic peak sign, and for CT the gloved finger Air bronchogram sign sign, CT halo sign, signet ring sign, comet tail sign, CT an- giogram sign, crazy paving pattern, tree-in-bud sign, feeding Bronchi, which are not normally seen, become visible as a result of vessel sign, split pleura sign, and reversed halo sign. opacification of the lung parenchyma. -

Eponyms in Radiologic Signs
Eponyms in radiologic signs Poster No.: C-0133 Congress: ECR 2014 Type: Educational Exhibit Authors: D. Andrade, L. Andrade, M. Magalhaes, L. Curvo-Semedo, F. Caseiro Alves; Coimbra/PT Keywords: Diagnostic procedure, Fluoroscopy, CT, Conventional radiography, Thorax, Musculoskeletal system, Gastrointestinal tract, Education and training DOI: 10.1594/ecr2014/C-0133 Any information contained in this pdf file is automatically generated from digital material submitted to EPOS by third parties in the form of scientific presentations. References to any names, marks, products, or services of third parties or hypertext links to third- party sites or information are provided solely as a convenience to you and do not in any way constitute or imply ECR's endorsement, sponsorship or recommendation of the third party, information, product or service. ECR is not responsible for the content of these pages and does not make any representations regarding the content or accuracy of material in this file. As per copyright regulations, any unauthorised use of the material or parts thereof as well as commercial reproduction or multiple distribution by any traditional or electronically based reproduction/publication method ist strictly prohibited. You agree to defend, indemnify, and hold ECR harmless from and against any and all claims, damages, costs, and expenses, including attorneys' fees, arising from or related to your use of these pages. Please note: Links to movies, ppt slideshows and any other multimedia files are not available in the pdf version of presentations. www.myESR.org Page 1 of 43 Learning objectives 1. To recognize the most frequent and important radiologic signs that are eponyms. -

Pulmonary Embolism: Diagnosis and Treatment
Pulmonary Embolism: Diagnosis and Treatment W. B. Davis No Disclosures www.chestpubs.org A negative D-dimer can exclude PE in low risk patients? 1. True 2. False Which of the following would you not use as sole initial therapy in PE? 1. IV unfractionated heparin 2. Rivaroxaban 3. Warfarin 4. Enoxaparin 5. Fondaparinux What is best treatment duration for PE provoked by surgery? 1. 3 months 2. 6 months 3. 12 months 4. lifetime Pulmonary Embolism • Common • Often fatal • Rapid diagnosis and treatment greatly reduce mortality PE is the great mimic of other pulmonary diseases • Sudden death • Atelectasis • Inferior MI • Pneumonia, uni- or • Acute asthma multilobar • Heart failure • Malignant pleural effusion • Radiographic paralyzed • Large rounded mass hemidiaphragm suggestive of lung • Hemoptysis suggestive cancer of bronchiectasis, lung • Long term dyspnea cancer or lung suggestive of COPD hemorrhage syndrome • Primary pulmonary hypertension Signs and Symptoms Not Helpful in Diagnosis • Dyspnea • Tachypnea • Pleuritic chest pain • Rales • Cough • Tachycardia • Hemoptysis • S4 • Loud S2P • Shock Wells Prediction Score ABG’s • Hypoxemia and respiratory alkalosis ABG’s • Hypoxemia and respiratory alkalosis • pO2 60 pCO2 32 pH 7.49 ABG’s • Hypoxemia and respiratory alkalosis • Can have normal pO2 ABG’s • Hypoxemia and respiratory alkalosis • Can have normal pO2 • Can have normal A-a DO2 ABG’s • Hypoxemia and respiratory alkalosis • Can have normal pO2 • Can have normal A-a DO2 • Not helpful in diagnosis BNP and Troponin • Lack sensitivity/specificity for PE BNP and Troponin • Lack sensitivity/specificity for PE • BNP or Troponin are associated with increased mortality Sinus Tachycardia EKG • Common • Massive PE – Precordial T wave inv. -

Common Pediatric Pulmonary Issues
Common Pediatric Pulmonary Issues Chris Woleben MD, FAAP Associate Dean, Student Affairs VCU School of Medicine Assistant Professor, Emergency Medicine and Pediatrics Objectives • Learn common causes of upper and lower airway disease in the pediatric population • Learn basic management skills for common pediatric pulmonary problems Upper Airway Disease • Extrathoracic structures • Pharynx, larynx, trachea • Stridor • Externally audible sound produced by turbulent flow through narrowed airway • Signifies partial airway obstruction • May be acute or chronic Remember Physics? Poiseuille’s Law Acute Stridor • Febrile • Laryngotracheitis (croup) • Retropharyngeal abscess • Epiglottitis • Bacterial tracheitis • Afebrile • Foreign body • Caustic or thermal airway injury • Angioedema Croup - Epidemiology • Usually 6 to 36 months old • Males > Females (3:2) • Fall / Winter predilection • Common causes: • Parainfluenza • RSV • Adenovirus • Influenza Croup - Pathophysiology • Begins with URI symptoms and fever • Infection spreads from nasopharynx to larynx and trachea • Subglottic mucosal swelling and secretions lead to narrowed airway • Development of barky, “seal-like” cough with inspiratory stridor • Symptoms worse at night Croup - Management • Keep child as calm as possible, usually sitting in parent’s lap • Humidified saline via nebulizer • Steroids (Dexamethasone 0.6 mg/kg) • Oral and IM route both acceptable • Racemic Epinephrine • <10kg: 0.25 mg via nebulizer • >10kg: 0.5 mg via nebulizer Croup – Management • Must observe for 4 hours after