Statistical Analysis Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

114.3 Cmr: Division of Health Care Finance and Policy Ambulatory Care
114.3 CMR: DIVISION OF HEALTH CARE FINANCE AND POLICY AMBULATORY CARE 114.3 CMR 40.00: RATES FOR SERVICES UNDER M.G.L. c. 152, WORKERS’ COMPENSATION ACT Section 40.01: General Provisions 40.02: General Definitions 40.03: Service and Rate Coverage Provisions 40.04: Provisions Affecting Eligible Providers 40.05: Policies for Individual Service Types 40.06: Fees 40.07: Appendices 40.08: Severability 40.01: General Provisions (1) Scope, Purpose and Effective Date. 114.3 CMR 40.00 governs the payment rates effective April 1, 2009 for purchasers of health care services under M.G.L. c. 152, the Workers’ Compensation Act. Payment rates for services provided by hospitals are set forth in 114.1 CMR 41.00. Program policies relating to medical necessity and clinical appropriateness are determined pursuant to M.G.L. c. 152 and 452 CMR 6.00. (2) Coverage. The payment rates set forth in 114.3 CMR 40.06 are full payment for services provided under M.G.L. c. 152, § 13, including any related administrative or overhead costs. The insurer, employer and health care service provider may agree upon a different payment rate for any service set forth in the fee schedule in 114.3 CMR 40.00. No employee may be held liable for the payment for health care services determined compensable under M.G.L. c. 152, § 13. (3) Administrative Bulletins. The Division may issue administrative bulletins to clarify substantive provisions of 114.3 CMR 40.00, or to publish procedure code updates and corrections. For coding updates and correction, the bulletin will list: (a) new code numbers for existing codes, with the corresponding cross references between existing and new codes numbers; (b) deleted codes for which there are no corresponding new codes; and (c) codes for entirely new services that require pricing. -

Orthopaedics AMPUTATION PRE-APPROVAL CODE DESCRIPTION REQUIRED PAYMENT INDICATORS PAYMENT RULES
Schedule of Benefits for Professional Fees 2020 Orthopaedics AMPUTATION PRE-APPROVAL CODE DESCRIPTION REQUIRED PAYMENT INDICATORS PAYMENT RULES Amputation, finger or thumb, primary or secondary, any joint or phalanx, single, including 3140 neurectomies; with direct closure (use also for traumatic amputations) No 3145 Amputation of two or more fingers No 3280 Amputation through forearm No 3415 Amputation through arm No 3464 Fore quarter amputation No 3645 Above knee amputation No 3690 Hind quarter amputation No 3790 Below knee amputation No 4255 Trans metatarsal amputation of foot No 4260 Trans metatarsal amputation of one toe No 4261 Trans metatarsal amputation of two or more toes No 4330 Trimming of stump following amputation of limb No ANKLE PRE-APPROVAL CODE DESCRIPTION REQUIRED PAYMENT INDICATORS PAYMENT RULES 3955 Arthrodesis of ankle joint No Arthroscopy, ankle, with or without removal of loose body or foreign body, with or without 3956 synovectomy, debridement (I.P.) No Independent Procedure, Day Care Arthroscopy, ankle, surgical, excision of osteochondral defect of talus and/ or tibia, including 3961 drilling of the defect (I.P.) No Independent Procedure 1 Night Only Arthroscopically aided repair of large osteochondritis dissecans lesion, talar dome fracture, or 3962 tibial plafond fracture, with or without internal fixation (includes arthroscopy) (I.P.) No Independent Procedure 3963 Arthroscopy, subtalar joint, surgical, with subtalar arthrodesis (I.P.) No Independent Procedure 3965 Fracture of medial or lateral malleolus (1st -

Talectomy (Astragalectomy) and Tibiocalcaneal Arthrodesis Following Traumatic Talus Fracture-Dislocation
Talectomy (astragalectomy) and tibiocalcaneal arthrodesis following traumatic talus fracture-dislocation The Foot and Ankle Online Journal 12 (2): 4 1 by Dr Alison Zander, MBBCh, BSc (hons), MSc (PHNutr) , Mr Anirudh Gadgil, MBBS, M.S. (Orth), 2 3* FRCS (Ed), FRCS (Trauma & Ortho) , Derek Protheroe, BSc(Hons), MSc, PgDip Talus fractures occur rarely but are often associated with complications and functional limitations. Urgent reduction of associated dislocations is recommended with open-reduction and internal fixation of displaced fractures when adjacent soft tissue injury permits [1]. However, it is important to remember that there is a high incidence of long term complications, along with a significant impact on activities of daily living and quality of life. This case report describes the successful treatment of a severely comminuted talar fracture dislocation with primary talectomy and tibio-calcaneal arthrodesis. A reminder that in selected cases that the talectomy (astragalectomy) may be a viable alternative. Keywords: talus, comminuted, tibiocalcaneal arthrodesis, fusion, talectomy, astragalectomy, trauma, avascular-necrosis, AVN This is an Open Access article distributed under the terms of the Creative Commons Attribution License. It permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ©The Foot and Ankle Online Journal (www.faoj.org), 2019. All rights reserved. alus fractures account for less than 1% of all surfaces is precluded secondary to comminution [4]. fractures, they may be caused by high-energy The talus is the second largest of the tarsal bones, trauma, and any other form of forced with more than half of its surface being covered with dorsiflexion injury to the ankle and foot [1]. -

TIBIOTALOCALCANEAL and PANTALAR ARTHRODESIS Foot
TIBIOTALOCALCANEAL AND PANTALAR ARTHRODESIS Foot and Ankle Clinics Author: George E. Quill, Jr., M.D. Since the report by Albert in 1882, arthrodesis has been an accepted treatment for painful arthritis of the ankle and subtalar joints.3 For the purpose of this and other orthopaedic articles, surgical fusion of the ankle (tibiotalar) and subtalar (talocalcaneal) joints at the same operative sitting will be termed tibiotalocalcaneal arthrodesis. Fusion of the talus to all the bones articulating with it (distal tibia, calcaneus, tarsonavicular, and cuboid) is termed pantalar arthrodesis.13,20 Numerous techniques exist describing fusion approaches and implants, all having in common a similar goal: a solid, pain free arthrodesis in a biomechanically stable and functional position.1-31 The indications for tibiotalocalcaneal arthrodesis include avascular necrosis of the talus or a failed total ankle arthroplasty with subtalar intrusion.14,16,20,21 The failed ankle fusion with insufficient talar body, as well as patients with rheumatoid or osteoarthritic involvement of these joints are also candidates for tibiotalocalcaneal fusion.8,16,23,25,30 Other indications include the sequelae of trauma and the severe deformity of untreated clubfoot and neuromuscular disease.5,7,20,23,24 Patients with Charcot arthropathy, skeletal defects after tumor reconstruction, pseudarthrosis, as well as fixed and various flail deformities about the hindfoot and ankle are also candidates for tibiotalocalcaneal and, in certain instances, pantalar arthrodesis.4,9,18,20,22,27,28 -

Mississippi Division of Medicaid AMBULATORY SURGICAL CENTERS (ASC) FEE SCHEDULE COVER SHEET
Mississippi Division of Medicaid AMBULATORY SURGICAL CENTERS (ASC) FEE SCHEDULE COVER SHEET Additional References: MS Division of Medicaid Website MS Envision Interactive Fee Schedule MS Envision Downloadable Fee Schedule Medicaid National Correct Coding Initiative (NCCI) Edits Note Number Column Title Details • Healthcare Common Procedure Coding System (HCPCS) or Current Procedural 1 Code Terminology (CPT) Code • Short Descriptor for the Healthcare Common Procedure Coding System (HCPCS) or 2 Description Current Procedural Terminology Code Clinical Description 3 Min Age • This column is the covered minimum age for the service. 4 Max Age • This column is the covered maximum age for the service. • This column represents the begin date of which the fee in columns H became effective. 5 Begin Date 6 End Date • This column represents the end date of the fee segment in columns H. • This column represents the maximum units the Division of Medicaid covers for the 7 Max Units service. • This column is the maximum amount that Division of Medicaid will pay for each unit. 8 Fee • When the is maximum fee is listed as 0.00, the service is a packaged service/item, no separate payment made. • This column is the maximum amount less the 5% reduction required by Miss. Code Ann. §43-13-117(B) that the Division of Medicaid will pay for each unit. 9 Fee Reduced • When the maximum fee is listed as 0.00, the service is a packaged service/item, no separate payment made. Mississippi Division of Medicaid AMBULATORY SURGICAL CENTERS (ASC) WEBSITE FEE SCHEDULE Print Date: JULY 1, 2021 The fee schedules located on the Mississippi Medicaid website are prepared to assist Medicaid providers and are not intended to grant rights or impose obligations. -

Introduction
Introduction Pursuant to Mississippi Code Annotated (MCA), section 71-3-15(3)(Rev. 2000), the following Fee Schedule, including Cost Containment and Utilization Management rules and guidelines, is hereby established in order to implement a medical cost containment program. This Fee Schedule, and accompanying rules and guidelines, applies to medical services rendered after the effective date of August 1, 2008, July 1, 2010, and, in the case of inpatient treatment, to services where the discharge date is on or after August 1, 2008 July 1, 2010. This Fee Schedule establishes the maximum level of medical and surgical reimbursement for the treatment of work-related injuries and/or illnesses, which the Mississippi Workers’ Compensation Commission deems to be fair and reasonable. This Fee Schedule shall be used by the Workers’ Compensation Commission, insurance payers, and self- insurers for approving and paying medical charges of physicians, surgeons, and other health care providers for services rendered under the Mississippi Workers’ Compensation Law. This Fee Schedule applies to all medical services provided to injured workers by physicians, and also covers other medical services arranged for by a physician. In practical terms, this means professional services provided by hospital-employed physicians, as well as those physicians practicing independently, are reimbursed under this Fee Schedule. The Commission will require the use of the most current version of the CPT book and HCPCS codes and modifiers in effect at the time services are rendered. All coding, billing and other issues, including disputes, associated with a claim, shall be determined in accordance with the CPT rules and guidelines in effect at the time service is rendered, unless otherwise provided in this Fee Schedule or by the Commission. -

Products of Ambulatory Surgery 2008 Procedure Codes
Products of Ambulatory Surgery 2008 Procedure Codes CPT Begin /HCPCS CPT Description PAS PAS Description Date 28035 Release, tarsal tunnel (posterior tibial nerve decompression) 1 Nerve Repair 2/1/2004 28080 Excision, interdigital (Morton) neuroma, single, each 1 Nerve Repair 2/1/2004 61790 Creation of lesion by stereotactic method, percutaneous, by neurolytic agent (eg, alcohol, thermal, electrical, radiofrequency); gasserian ganglion 1 Nerve Repair 2/1/2004 Insertion or replacement of cranial neurostimulator pulse generator or receiver, direct or inductive coupling; with connection to a single electrode 61885 array 1 Nerve Repair 2/1/2004 InIncisioncision aandnd subcutasubcutaneousneous pplacementlacement ooff ccranialranial nneurostimulatoreurostimulator pupulselse gegeneratornerator oorr rreceiver,eceiver, ddirectirect oorr ininductiveductive coupcoupling;ling; wiwithth coconnectionnnection to ttwowo oorr 61886 more electrode arrays 1 Nerve Repair 2/1/2004 62268 Percutaneous aspiration, spinal cord cyst or syrinx 1 Nerve Repair 2/1/2004 62269 Biopsy of spinal cord, percutaneous needle 1 Nerve Repair 2/1/2004 62270 Spinal puncture, lumbar, diagnostic 1 Nerve Repair 2/1/2004 62272 Spinal puncture, therapeutic, for drainage of cerebrospinal fluid (by needle or catheter) 1 Nerve Repair 2/1/2004 62273 Injection, epidural, of blood or clot patch 1 Nerve Repair 2/1/2004 62280 Injection/infusion of neurolytic substance (eg, alcohol, phenol, iced saline solutions), with or without other therapeutic substance; subarachnoid 1 Nerve Repair 2/1/2004 -

Department of Veterans Affairs § 4.71A
Department of Veterans Affairs § 4.71a [29 FR 6718, May 22, 1964, as amended at 43 FR 45349, Oct. 2, 1978] § 4.71a Schedule of ratings—musculo- ACUTE, SUBACUTE, OR CHRONIC DISEASES— skeletal system. Continued ACUTE, SUBACUTE, OR CHRONIC DISEASES Rat- ing Rat- NOTE (1): A rating of 10 percent, as an exception ing to the amputation rule, is to be assigned in any case of active osteomyelitis where the 5000 Osteomyelitis, acute, subacute, or chronic: amputation rating for the affected part is no Of the pelvis, vertebrae, or extending into major percent. This 10 percent rating and the other joints, or with multiple localization or with long partial ratings of 30 percent or less are to be history of intractability and debility, anemia, combined with ratings for ankylosis, limited amyloid liver changes, or other continuous motion, nonunion or malunion, shortening, constitutional symptoms ................................... 100 etc., subject, of course, to the amputation rule. Frequent episodes, with constitutional symptoms 60 The 60 percent rating, as it is based on con- With definite involucrum or sequestrum, with or stitutional symptoms, is not subject to the am- without discharging sinus ................................. 30 putation rule. A rating for osteomyelitis will not With discharging sinus or other evidence of ac- be applied following cure by removal or radical tive infection within the past 5 years ................ 20 resection of the affected bone. Inactive, following repeated episodes, without evidence of active infection in past 5 years ..... 10 367 VerDate 0ct<02>2002 14:22 Oct 08, 2002 Jkt 197134 PO 00000 Frm 00367 Fmt 8010 Sfmt 8010 Y:\SGML\197134T.XXX 197134T EC04NO91.002</GPH> § 4.71a 38 CFR Ch. -

Case Log Guidelines for Foot and Ankle Orthopaedic Surgery Review Committee for Orthopaedic Surgery
Case Log Guidelines for Foot and Ankle Orthopaedic Surgery Review Committee for Orthopaedic Surgery The ACGME Case Log System for Foot and Ankle Orthopaedic Surgery allows fellows to document their operative experience during the 12-month educational program and enables the Review Committee to monitor programs to ensure that fellows have an adequate volume and variety of experiences appropriate for fellowship education. In the past, fellows used the Case Log System designed for the core residency program to report their cases. The Review Committee has now identified defined case categories (listed here on the following pages) as appropriate for the focused education of foot and ankle orthopaedic surgery fellows; the ACGME Case Log System for Foot and Ankle Orthopaedic Surgery has been revised to identify the CPT codes tracked to each defined case category. The CPT codes available in each category are listed; note that fellows are NOT expected to report cases using all listed CPT codes. While it is expected that fellows will report cases in each defined case category, there are no minimum case numbers required at this time. The new system is in place now. To log a case, fellows will continue to identify the patient type (adult or pediatric), and must also identify their role in the case as either Level 1 (primary or supervising fellow surgeon) or Level 2 (assisting fellow surgeon). All procedures at both levels require appropriate faculty member supervision and participation in the case. At this time, both Level 1 and Level 2 participation will count. In addition, fellows must identify a primary CPT code for each case, but should include all additional CPT codes as appropriate. -

STATISTICAL ANALYSIS PLAN Study Title
STATISTICAL ANALYSIS PLAN Study Title: A Phase 3, Randomized, Double-Blind Study to Evaluate the Efficacy and Safety of Switching from Tenofovir Disoproxil Fumarate (TDF) 300 mg QD to Tenofovir Alafenamide (TAF) 25 QD in Subjects with Chronic Hepatitis B who are Virologically Suppressed Name of Test Drug: Tenofovir Alafenamide (TAF) Study Number: GS-US-320-4018 Protocol Version (Date): Amendment 1 (21 December 2016) Analysis Type: Week 48 Analysis Analysis Plan Version: 1.0 Analysis Plan Date: 17 October 2018 Analysis Plan Author(s): PPD CONFIDENTIAL AND PROPRIETARY INFORMATION TABLE OF CONTENTS STATISTICAL ANALYSIS PLAN..............................................................................................................................1 TABLE OF CONTENTS ..............................................................................................................................................2 LIST OF IN- TEXT TABLES.......................................................................................................................................4 LIST OF IN- TEXT FIGURES .....................................................................................................................................4 LIST OF ABBREVIATIONS........................................................................................................................................5 1. INTRODUCTION ................................................................................................................................................8 1.1. -
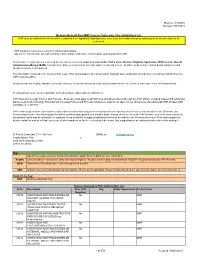
MMP Services That Require Prior Auth List 10-01-2018.Xlsx
Effective: 9/13/2018 Revised: 10/01/2018 Medicare-Medicaid Plan (MMP) Services that require Prior Authorization List HAP must be notified when the member is admitted for all inpatient hospitalizations, even if you have obtained a prior authorization for the procedure to be performed. • HAP members must receive care from contracted providers. • Any service listed below, when provided by a non-contracted provider, requires prior authorization from HAP. To determine if a procedure is a covered benefit and meets criteria, providers must utilize HAP's online Member Eligibility Application (MEA) and the Benefit Administration Manual (BAM). It is imperative that you verify benefit coverage prior to rendering service, as failure to do so may result in denial of payment and Members must be held harmless. The information contained in the Services that require Prior Authorization List is protected by copyright laws. Duplication should occur only with permission from the HAP Corporate Office. Providers shall not modify, translate, decompile, disclose, create nor attempt to create any derivative work in the Services that require Prior Authorization List. Treating providers are solely responsible for medical advice and treatment of Members. HAP’s Benefit Coverage Policies and Procedure Reference Lists apply to all HAP lines of business offered through any HAP affiliate including insured and self-funded plans except for the following: These Benefit Coverage Policies and Procedure Reference Lists do not apply to lines of business offered through HAP affiliates ASR and Midwest Health Plan. HAP continuously reviews and monitors all procedures to determine any potential changes of coverage that would affect current procedure lists. -
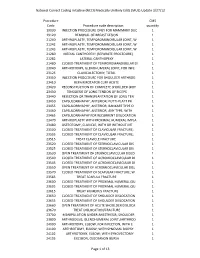
Medically Unlikely Edits (MUE) Update 3/27/12
National Correct Coding Initiative (NCCI) Medically Unlikely Edits (MUE) Update 3/27/12 Procedure CMS Code Procedure code description quantity 19030 INJECTION PROCEDURE ONLY FOR MAMMARY DUC 1 19120 REMOVAL OF BREAST LESION 1 21240 ARTHROPLASTY, TEMPOROMANDIBULAR JOINT, W 1 21242 ARTHROPLASTY, TEMPOROMANDIBULAR JOINT, W 1 21243 ARTHROPLASTY, TEMPOROMANDIBULAR JOINT, W 1 21280 MEDIAL CANTHOPEXY (SEPARATE PROCEDURE) 1 21282 LATERAL CANTHOPEXY 1 21480 CLOSED TREATMENT OF TEMPOROMANDIBULAR DI 1 23040 ARTHROTOMY, GLENOHUMERAL JOINT, FOR INFE 1 23125 CLAVICULECTOMY; TOTAL 1 23350 INJECTION PROCEDURE FOR SHOULDER ARTHROG 1 23410 REPAIR ROTATOR CUFF ACUTE 1 23420 RECONSTRUCTION OF COMPLETE SHOULDER (ROT 1 23430 TENODESIS OF LONG TENDON OF BICEPS 1 23440 RESECTION OR TRANSPLANTATION OF LONG TEN 1 23450 CAPSULORRHAPHY, ANTERIOR; PUTTI-PLATT PR 1 23455 CAPSULORRHAPHY, ANTERIOR; BANKART TYPE O 1 23460 CAPSULORRHAPHY, ANTERIOR, ANY TYPE; WITH 1 23465 CAPSULORRHAPHY FOR RECURRENT DISLOCATION 1 23470 ARTHROPLASTY WITH PROXIMAL HUMERAL IMPLA 1 23480 OSTEOTOMY, CLAVICLE, WITH OR WITHOUT INT 1 23500 CLOSED TREATMENT OF CLAVICULAR FRACTURE; 1 23505 CLOSED TREATMENT OF CLAVICULAR FRACTURE; 1 23515 TREAT CLAVICLE FRACTURE 1 23520 CLOSED TREATMENT OF STERNOCLAVICULAR DIS 1 23525 CLOSED TREATMENT OF STERNOCLAVICULAR DIS 1 23530 OPEN TREATMENT OF STERNOCLAVICULAR DISLO 1 23540 CLOSED TREATMENT OF ACROMIOCLAVICULAR DI 1 23545 CLOSED TREATMENT OF ACROMIOCLAVICULAR DI 1 23550 OPEN TREATMENT OF ACROMIOCLAVICULAR DISL 1 23570 CLOSED TREATMENT OF SCAPULAR FRACTURE;