Hyperhidrosis: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PRIMARY HYPERHIDROSIS Prevalence and Impacts for the Individual
PRIMARY HYPERHIDROSIS Prevalence and impacts for the individual Alexander Shayesteh Afshar Department of Public Health and Clinical Medicine Dermatology and Venereology Umeå 2018 Copyright © Alexander Shayesteh Afshar 2018 This work is protected by the Swedish Copyright Legislation (Act 1960:729) Dissertation for PhD ISBN: 978-91-7601-822-4 ISSN: 0346-6612 New Series No 1940 Cover art: “Drop Beads” by Grant Ware and Alexander Shayesteh Afshar Electronic version available at: http://umu.diva-portal.org/ Printed by: Umu Print Service Umeå, Sweden 2018 To Ladan, Gabriel and Isabell In medicine we ought to know the causes of sickness and health. And because health and sickness and their causes are sometimes manifest, and sometimes hidden and not to be comprehended except by the study of symptoms, we must also study the symptoms of health and disease. Avicenna 973-1037 CE Table of contents Abstract ............................................................................................ iii Abbreviations .................................................................................... v Sammanfattning på svenska ............................................................ vi List of papers .................................................................................. vii Introduction ....................................................................................... 1 Sweat ................................................................................................................................. 1 Sweat glands .................................................................................................................... -

Unilateral Hyperhidrosis Associated with Underlying Intrathoracic Neoplasia
Thorax: first published as 10.1136/thx.41.10.814 on 1 October 1986. Downloaded from Thorax 1986;41:814-815 Unilateral hyperhidrosis associated with underlying intrathoracic neoplasia D C LINDSAY, J G FREEMAN, C 0 RECORD From the Department ofMedicine, Royal Victoria Infirmary, and University ofNewcastle upon Tyne Intrathoracic neoplasia is notable for the many ways in wall. There were metastatic plaques in the right hemithorax which it may present. We would like to report two cases and abdominal cavity but no evidence of metastases in the demonstrating a rare association between unilateral local- brain or the spinal cord. ised hyperhidrosis of the thoracic cage and underlying intra- thoracic neoplasm. Discussion Case reports The association of intrathoracic malignancy with sym- pathetic neurological complications, especially Homer's CASE 1 syndrome, is well recognised, particularly in the case of A 67 year old retired shotblaster complained ofa 3 kg weight tumours occurring at the thoracic inlet. Unilateral hyper- loss, mild dyspnoea, chest pain localised to the right costal hidrosis is an unusual phenomenon which has been reported margin, and profuse sweating localised to an area below the sporadically in association with various conditions, includ- right scapula. He smoked 15 cigarettes per day. Examination ing intracranial malignancy, encephalitis, syringomyelia, confirmed a right sided localised band of sweating at the trauma, neuritis, cervical rib, osteoma of the dorsal spine, level ofT6-9 posteriorly. Apart from minimal winging ofthe and chickenpox; in several cases no obvious underlying right scapula and some wasting of the right suprascapular cause has been evident. muscles no abnormal neurological signs were detected. -

Treatment of Hyperhidrosis Dr
“ Finding a solution to my sweating problem has With advanced technology and skilled hands, wholly changed my life. After having the Botox Matthew R. Kelleher, MD provides a full Premier Dermatology spectrum of services and procedures, including: for hyperhidrosis treatment, I am a thousand • Liposculpture times more confi dent and no longer afraid to • Botox, Juvéderm®, and Voluma™ Treatment TREATMENT OF lift my arms and be completely myself. I am so of Wrinkles thankful that this treatment exists!” • Laser Removal of Age Spots and Freckles HYPERHIDROSIS • Laser Facial Rejuvenation - Olivia • Laser Hair Removal Botox for hyperhidrosis patient • Laser Treatments of Rosacea, Facial Redness, and Spider Veins • Laser Scar Reduction • Laser Treatment of Stretch Marks “ Suffering from axillary hyperhidrosis, I thought • Laser Tattoo Removal • Laser Removal of Vascular Birthmarks there was nothing I could do. My condition • Laser and Photodynamic Treatment of Acne made me reluctant to participate in any social • Sclerotherapy for Leg Veins environment. Every day was a struggle until • Thermage® Radiofrequency Tissue Tightening liposuction for hyperhidrosis changed my life! • Microdermabrasion • Botox and Liposculpture Treatment of Hyperhidrosis Dr. Kelleher gave me the confi dence to feel • Sculpsure and Kybella for nonsurgical body sculpting comfortable in my own skin, and I never have to worry about embarrassing sweat stains again!” - Matthew Liposculpture for hyperhidrosis of the underarms patient “ After dealing with my excessive sweating for many years, without fully understanding it was a medical condition, Dr. Kelleher took the time to explain the treatment options available along with their results. I experienced immediate, positive results after my fi rst treatment which gave me a new sense of confi dence and removed the insurmountable stress I carried daily. -

Anorexia/Cachexia Heart Failure Symptom Management Guideline for Adults, Age 19 and Older in British Columbia
Anorexia/Cachexia Heart Failure Symptom Management Guideline For adults, age 19 and older in British Columbia What is anorexia? Anorexia is a syndrome characterized by some or all of the following symptoms: loss of appetite, nausea, early satiety, weakness, fatigue, food aversion, and significant physical and/or psychological symptoms. Causes of anorexia are multifactorial and include fatigue, dyspnea, medication side-effects, nausea, depression, anxiety and sodium restricted diets, which may all be found in patients with heart failure. What is cachexia? Cachexia is a syndrome characterized by severe body weight, fat and muscle loss and increased protein catabolism due to underlying disease. The prevalence of cachexia is 16–42% in the heart failure population and is associated with a 50%, 18 month mortality risk independent of variables such as ejection fraction, age and functional ability. How is cachexia diagnosed? Chronic condition with >5% weight loss in <12 months; or body mass index (BMI) <20kg/m2; and 3 out of 5 additional criteria: 1) Fatigue, 2) Decreased muscle strength, 3) Anorexia, 4) Low muscle mass, 5) Abnormal biochemistry *Blood testing to diagnose cachexia in advanced stages of disease is not advocated. Reminder: Malnutrition also affects prognosis in patients with heart failure and is often found in early transitions of the disease. However this symptom management guideline will focus on the assessment and treatment of anorexia and cachexia. Approach to Managing Anorexia/Cachexia Assessment History: When did weight loss begin? How much weight was lost? Obtain baseline (dry) weight. How is [the patients] appetite? What do they eat or drink on a typical day? How has weight loss affected mood? Ask about: nausea, early satiety, dyspnea, poor oral hygiene, dysphagia, malabsorption, bowel habits. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Cancer Cachexia and Fatigue
CME Palliative care Cancer cachexia and mechanisms (Fig 1). The cachectic Other cachectic factors patient is analogous to an accelerating Cachexia can occur in the absence of car running out of petrol. The anorexia anorexia, suggesting that catabolic fatigue component of cancer cachexia reduces mediators produced by tumour or host fuel supply (by ca 300–500 kcal/day) cells are involved in the cancer cachexia whilst accelerated metabolic cycling Grant D Stewart BSc(Hons) MBChB MRCS(Ed), process.9 Experimental cachexia models drives hypermetabolism (by ca Surgical Research Fellow suggest pro-inflammatory cytokines, 100–200 kcal/day). There are also the Richard JE Skipworth BSc(Hons) MBChB such as tumour necrosis factor- , inter- direct catabolic effects of muscle proteol- α MRCS(Ed), Surgical Research Fellow leukin (IL)-6, IL-1 and interferon- , can ysis and lipolysis. These changes underlie γ Kenneth CH Fearon MBChB(Hons) MD all play a role. Activation of the neuro- a key paradox of cachexia: whilst meta- FRCS(Glas) FRCS(Ed) FRCS(Eng), Professor of endocrine stress response is also thought bolic rate may be increased, overall (or Surgical Oncology to be important. Potential mediators total) energy expenditure is decreased Department of Clinical and Surgical Sciences include increased adrenergic activity, ele- due to a fall in physical activity.7 (Surgery), University of Edinburgh, Royal vated cortisol, low insulin and increased Infirmary, Edinburgh activity of the renin-angiotensin system.1 Anorexia With regard to tumour-specific Clin Med 2006;6:140–3 The anorexia component of cancer cachectic factors, proteolysis-inducing cachexia has both a neurohumoral mech- factor (PIF) is produced by tumours and anism due to disturbance of the central excreted in the urine of patients with Background physiological mechanisms controlling cancer cachexia. -

Workforce Prevention and Influenza Illness Policy Guidelines
Workforce Prevention and Influenza Illness Policy Guidelines This document provides guidance to (Company) _________________ supervisors and employees on how to handle influenza-like illness in the (Company) _________________ workplace. OVERVIEW Influenza is a respiratory disease caused by the influenza virus. Influenza is spread primarily person-to-person through coughing, sneezing, or nasal secretions from infected people, or when someone touches something with flu viruses on it before touching their mouths or noses. Infected people can spread the virus to others even before symptoms develop, and can be contagious for several days after becoming sick. Employees experiencing flu-like symptoms such as fever and chills with cough, sore throat, head and muscle ache, nasal congestion and fatigue should not come to work or should leave work to go home. Employees should stay home and avoid contact with other people until they have no symptoms for 24 hours without medication. Employees can plan to return to work 24 hours after fever subsides, without use of fever lowering medications. Supervisors are responsible for ensuring their staff members to stay away from work when experiencing influenza-like illness. Employees have a duty to practice healthy hygiene habits to prevent the spread of disease, and an expectation of working in an environment free of influenza-like illness. Those with severe symptoms, such as difficulty breathing, or at higher risk for complication from influenza should call their health care provider. If you are an employee who is experiencing flu-like symptoms: • Inform your supervisor that you are experiencing flu-like symptoms and leave the workplace as soon as possible. -

Association of Central Hypersomnia and Fatigue in Patients with Multiple Sclerosis: a Polysomnographic Study
Published Ahead of Print on April 30, 2021 as 10.1212/WNL.0000000000012120 Neurology Publish Ahead of Print DOI: 10.1212/WNL.0000000000012120 Association of Central Hypersomnia and Fatigue in Patients With Multiple Sclerosis: A Polysomnographic Study Author(s): 1,2 3 4 Anne-Laure Dubessy, MD, PhD ; Sophie Tezenas du Montcel, MD, PhD ; Frederique Viala, MD ; 5 6 5 Rana ASSOUAD, MD ; Michel Tiberge, MD ; caroline papeix, MD, PhD ; catherine Lubetzki, MD, 5,7 4 2,7 1,7 PhD ; Michel Clanet, MD, PhD ; Isabelle Arnulf, MD, PhD ; Bruno Stankoff, MD, PhD Equal Author Contributions: Isabelle Arnulf and Bruno Stankoff contributed equally to this work Corresponding Author: Anne-Laure Dubessy [email protected] Affiliation Information for All Authors: 1. Neurology Department, Saint Antoine Hospital, AP-HP, Paris; 2. Sleep Disorders Unit and National Reference Center for Narcolepsy and Hypersomnia Pitié- Salpêtrière University Hospital, APHP, Paris; 3.Department of Biostatistics, Pitié-Salpêtrière University Hospital, APHP; 4. Neurology Department, Purpan Hospital, Toulouse, France; 5. Neurology Department, Pitié-Salpêtrière University Hospital, APHP, Paris; 6. Neurophysiology Department, Purpan Hospital, Toulouse; 7. Sorbonne Université, Institut du Cerveau et de la Moelle épinière, ICM, Hôpital de la Pitié Salpêtrière, Inserm UMR S 1127, CNRS UMR 7225, Paris Neurology® Published Ahead of Print articles have been peer reviewed and accepted for publication. This manuscript will be published in its final form after copyediting, page composition, -

NORIG 2BC - Blood Collection for DNABC109 Mar 09 BC - Blood Collection for DNA
Keyed: ( ) NORIG 2BC - Blood Collection for DNABC109 Mar 09 BC - Blood Collection for DNA Purpose: Document the collection of whole blood for shipment to NIDDK Genetics Repository at Rutgers University for DNA extraction and banking. Complete this form only if the patient signed the consent for genetic research. When: Screening visit s1 or s2 and as needed during follow-up due to a low yield (less than 50 μg) of DNA (during follow-up, use the visit code of the follow-up visit that is open). By whom: Clinical Coordinator and laboratory personnel responsible for collection of whole blood. Instructions: (1) Apply MACO labels specific for the patient and visit to the EDTA vacutainer tubes; these labels are generated by the clinical center upon registration (screening labels) or after randomization (follow-up visit labels). Affix duplicate tube label in item 13. (2) Fill two 10 mL EDTA vacutainer tubes with whole blood (see SOP I, section 6.2). (3) Pack the whole blood tubes in the specimen shippers supplied by the NIDDK Genetics Repository and ship to the NIDDK Genetic Repository at Rutgers University on the same day blood is collected. Ship blood at ambient room temperature. Use the preprinted Federal Express shipping label, marked for Priority Overnight Delivery, to ship whole blood to the NIDDK Genetics Repository Monday-Friday. A. Center, patient and visit identification C. Specimen for Genetics Repository 1. Center code: Attach MACO labels to two 10mL EDTA tubes and fill each with whole blood; invert each tube gently 6 times to mix blood with additives; keep tubes at 2. -
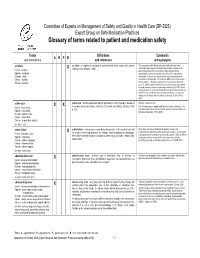
Glossary of Terms Related to Patient and Medication Safety
Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices Glossary of terms related to patient and medication safety Terms Definitions Comments A R P B and translations and references and synonyms accident accident : an unplanned, unexpected, and undesired event, usually with adverse “For many years safety officials and public health authorities have Xconsequences (Senders, 1994). discouraged use of the word "accident" when it refers to injuries or the French : accident events that produce them. An accident is often understood to be Spanish : accidente unpredictable -a chance occurrence or an "act of God"- and therefore German : Unfall unavoidable. However, most injuries and their precipitating events are Italiano : incidente predictable and preventable. That is why the BMJ has decided to ban the Slovene : nesreča word accident. (…) Purging a common term from our lexicon will not be easy. "Accident" remains entrenched in lay and medical discourse and will no doubt continue to appear in manuscripts submitted to the BMJ. We are asking our editors to be vigilant in detecting and rejecting inappropriate use of the "A" word, and we trust that our readers will keep us on our toes by alerting us to instances when "accidents" slip through.” (Davis & Pless, 2001) active error X X active error : an error associated with the performance of the ‘front-line’ operator of Synonym : sharp-end error French : erreur active a complex system and whose effects are felt almost immediately. (Reason, 1990, This definition has been slightly modified by the Institute of Medicine : “an p.173) error that occurs at the level of the frontline operator and whose effects are Spanish : error activo felt almost immediately.” (Kohn, 2000) German : aktiver Fehler Italiano : errore attivo Slovene : neposredna napaka see also : error active failure active failures : actions or processes during the provision of direct patient care that Since failure is a term not defined in the glossary, its use is not X recommended. -

Hyperhidrosis Hyperhidrosis Is a Condition Characterised by Abnormally Increased Sweating, in Excess of That Required for Regulation of Body Temperature
20 Dermatology Hyperhidrosis Hyperhidrosis is a condition characterised by abnormally increased sweating, in excess of that required for regulation of body temperature. Hyperhidrosis can either be generalised or localised to specifc parts of the body, such as hands, feet and axillae. Hyperhidrosis can be divided into primary or idiopathic, and a secondary type. Te primary type usually starts during adolescence or even earlier, while secondary hyperhidrosis can start at any point in life. Te latter form may be due to a disorder of the thyroid or pituitary gland, diabetes mellitus, tumours, gout, menopause, or certain medications. Tis article highlights the clinical features and the treatment options for this condition. Nabil Aly, Consultant Physician, University Hospital Aintree, Liverpool email [email protected] Epidemiology Hyperhidrosis is sweating in axillae; localised hyperhidrosis, excess of that required for and generalised hyperhidrosis.1,2 normal thermoregulation. It is a Localised hyperhidrosis, unlike People of all ages can be afected condition that usually begins in generalised hyperhidrosis, by hyperhidrosis. Primary either childhood or adolescence usually begins in childhood or hyperhidrosis affects men and can affect any site on the adolescence. Localised unilateral and women equally, and most body. However, the sites most or segmental hyperhidrosis is commonly occurs among people commonly afected are the palms, rare and of unknown origin. aged 25–64 years. Some may soles, and axillae. Excessive The condition usually presents have been affected since early sweating may be primary on the forearm or forehead in childhood, and about 30–50% (idiopathic) or secondary to otherwise healthy individuals, have another family member medication use, certain diseases, without evidence of the typical afflicted, implying a genetic metabolic disorders, or febrile triggering factors found in predisposition.5 Localised illnesses. -

Unilateral Hyperhidrosis Secondary to Brainstem Meningioma Producing Mass Effect
Volume 25 Number 11| November 2019| Dermatology Online Journal || Photo Vignette 25(11):9 Unilateral hyperhidrosis secondary to brainstem meningioma producing mass effect Ashlee Margheim1 BSN, Courtney R Schadt2 MD Affiliations: 1University of Louisville School of Medicine, Louisville, Kentucky, USA, 2Division of Dermatology, University of Louisville, Louisville, Kentucky, USA Corresponding Author: Ashlee Margheim, BSN, 3810 Springhurst Boulevard, Louisville, KY 40241, Tel: 502-572-4739, Email: [email protected] lesions of the hypothalamus, and cerebral or Abstract brainstem strokes [2, 3]. We report a case of a 61- Unilateral hyperhidrosis of neurological origin has year-old man with isolated sweating on the left been associated with head trauma, cerebral palsy, side of his entire body; a right-sided brainstem spinal cord injury, peripheral neuropathy, lesions meningioma producing mass effect is the of the hypothalamus, and cerebral or brainstem suspected underlying cause. strokes. In this report, we describe a 61-year-old man with isolated sweating on the left side of his entire body. A right-sided brainstem meningioma producing mass effect is suspected as the Case Synopsis underlying etiology. A 61-year-old right-handed male with no known medical history presented to the dermatologist Keywords: unilateral hyperhidrosis, meningioma with complaints of sweating on the left side of the body, including face, trunk, left arm, and left leg for a duration of one year. He reported sweating that drenched his clothes on occasion that was Introduction worse during the summer months. The patient Hyperhidrosis is defined as a condition of emphasized a clear line of demarcation between excessive sweating that exceeds the left and right sides of his body, with excessive thermoregulatory requirements.