Topical and Systemic Anticholinergic for Treating Hyperhidrosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office
3,092,548 United States Patent Office Patented June 4, 1963 2 are the various side effects that occur in an appreciable 3,092,548 number of patients, the foremost of which are a blurring METHOD OF TREATING PEPTICULCERS WITH PANTOTHENECACD of vision, drowsiness and a general dry condition mani Albert G. Worton, Columbus, Ohio, assignor to The fested by a retarded salivation, reduction of perspiration Warren-Teed Products Company, Columbus, Ohio, a 5 and diminished urinary output. Other side effects which corporation of Ohio occur in some cases are glaucoma, stimulation of the cen No Drawing. Filed Oct. 27, 1960, Ser. No. 65,256 trol nervous system and in severe cases cardiac and respi 5 Claims. (Ci. 67-55) ratory collapse. These side effects increase to Some de gree with the increase in dosage. Despite the side effects This invention relates to preparation adapted for use O these compounds are fairly selective and highly effective in treating disorders of the gastro-intestinal tract, more in decreasing the volume and acidity of gastric secretion particularly in treating peptic ulcer, both of the duodenal and in reducing gastrointestinal motility. and gastric type, for hyperacidity, hypertropic gastritis, Understandably, the main problem in the past has been splenic flexure syndrome, biliary dyskinesia (postchole to provide a dosage of anticholinergic drug which will cystectomy syndrome) and hiatal hernia or other condi achieve the most beneficial results possible and yet mini tions where anticholinergic effect, either spasmolytic or mize the undesired side effects. One of the objects of antisecretory is indicated or where antiuicerogenic effect this invention is to provide novel compositions containing is indicated. -

An Approach to the Patient with a Dry Mouth
MedicineToday 2014; 15(4): 30-37 PEER REVIEWED FEATURE 2 CPD POINTS An approach to the patient with a dry mouth Key points • The subjective complaint of ELHAM AFLAKI MD; TAHEREH ERFANI MD; NICHOLAS MANOLIOS MB BS(Hons), PhD, MD, FRACP, FRCPA; xerostomia needs to be MARK SCHIFTER FFD, RCSI(Oral Med), FRACDS(Oral Med) differentiated from true salivary hypofunction. Dry mouth is a common and disabling problem. After exclusion of treatable • Salivary hypofunction can significantly reduce quality causes, treatment is symptomatic to prevent the consequences of salivary of life through its adverse hypofunction, such as tooth decay and infection of the oral mucosa. effects on taste, mastication, swallowing, cleansing of the erostomia, or the subjective feeling of neuropathic-induced orofacial dysaesthesia) mouth, killing of microbes a dry mouth, is a common complaint. and psychological and psychiatric disorders, and speech. It is often a consequence of salivary such as anxiety and depression. • Salivary hypofunction is a hypofunction (hyposalivation), in substantive risk factor for X which there is objective evidence of reduced NORMAL SALIVA PRODUCTION dental caries, oral mucosal salivary output or qualitative changes in saliva. Under normal physiological conditions, the disease and infection, Typically, patients complain of oral dryness salivary glands produce 1000 to 1500 mL of particularly oral candidiasis. only when salivary secretion is reduced by more saliva daily as an ultrafiltrate from the circu- • Patients should be than half.1 As saliva has a crucial role in taste lating plasma. Therefore, simple dehydration investigated for contributory perception, mastication, swallowing, cleansing reduces saliva production. The parotid glands and underlying causes, of the mouth, killing of microbes and speech, are the major source of serous saliva (60 to 65% which include drugs and abnormalities in saliva production can signif- of total saliva volume), producing the stimu- rheumatological diseases. -

Muscarinic Acetylcholine Receptor
mAChR Muscarinic acetylcholine receptor mAChRs (muscarinic acetylcholine receptors) are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certainneurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibersin the parasympathetic nervous system. mAChRs are named as such because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpineand scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Acetylcholine (ACh) is a neurotransmitter found extensively in the brain and the autonomic ganglia. www.MedChemExpress.com 1 mAChR Inhibitors & Modulators (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate Cat. No.: HY-76772A Cat. No.: HY-76772B Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic receptor agonist, is a candidate therapeutic drug for receptor agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR The general pharmacol. properties of this drug on the The general pharmacol. properties of this drug on the gastrointestinal, urinary, and reproductive systems and other… gastrointestinal, urinary, and reproductive systems and other… Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10mM x 1mL in DMSO, Size: 10mM x 1mL in DMSO, 1 mg, 5 mg 1 mg, 5 mg AC260584 Aclidinium Bromide Cat. No.: HY-100336 (LAS 34273; LAS-W 330) Cat. -

Pilocarpine (Systemic) | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Pilocarpine (Systemic) This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Salagen Brand Names: Canada JAMP Pilocarpine; M-Pilocarpine; Salagen What is this drug used for? It is used to treat dry mouth. What do I need to tell my doctor BEFORE I take this drug? If you have an allergy to pilocarpine or any other part of this drug. If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: Asthma, glaucoma, liver disease, or swelling in parts of the eye. If you are breast-feeding or plan to breast-feed. This is not a list of all drugs or health problems that interact with this drug. Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure Pilocarpine (Systemic) 1/6 that it is safe for you to take this drug with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor. What are some things I need to know or do while I take this drug? Tell all of your health care providers that you take this drug. This includes your doctors, nurses, pharmacists, and dentists. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Effect of Diazepam and Hyoscine Butylbromide on Response to Secretin and Cholecystokinin-Pancreozymin in Man
Gut: first published as 10.1136/gut.17.5.351 on 1 May 1976. Downloaded from Gut, 1976, 17, 351-353 Effect of diazepam and hyoscine butylbromide on response to secretin and cholecystokinin-pancreozymin in man J. H. B. SAUNDERS, GUYA MASOERO, AND K. G. WORMSLEY' From the Department of Therapeutics, University of Dundee, Dundee SUMMARY Ten subjects received secretin and cholecystokinin or, in duplicate tests, the two hor- mones together with either diazepam or diazepam plus hyoscine butylbromide in order to determine whether these drugs, which are often used during retrograde endoscopic cannulation ofthe pancreatic duct, affect pancreatic and biliary secretion in response to the hormones. Diazepam with hyoscine butylbromide reduced the secretion of trypsin into the duodenum and delayed the appearance of both trypsin and bilirubin in duodenal aspirate. These effects must be taken into account when interpreting pancreatic and biliary responses measured during direct cannulation of the pancreatic duct. Endoscopic retrograde cannulation of the pancreatic vents in the antrum. Secretin (1 clinical unit/kg-h) duct has recently been used to measure pancreatic and cholecystokinin-pancreozymin (1 Ivy unit/ function by collecting the pancreatic juice directly kg-h) were administered for 45 minutes by continuous from the pancreatic duct during stimulation with intravenous infusion in 0 15 mol/1 sodium chloride. hormones et The exogenous (Cotton al., 1974; Gregg hormones had been purchased from the GIH http://gut.bmj.com/ et al., 1975; Robberecht et -

PROPANTHELINE BROMIDE Tablets USP Rx Only DESCRIPTION
PROPANTHELINE BROMIDE Tablets USP PRODUCT OVERVIEW: Propantheline Bromide TABLET, FILM COATED Rx only DESCRIPTION Each tablet for oral administration contains: Propantheline bromide.................................................. 15 mg Propantheline bromide, a synthetic quaternary ammonium compound, occurs as white or nearly white crystals. It is odorless and has a bitter taste, and is very soluble in water and chloroform; practically insoluble in ether, acetone and ethyl acetate. It is designated chemically as (2- Hydroxyethyl) diisopropylmethyl-ammonium bromide xanthene-9-carboxylate. The structural formula is: Inactive Ingredients The tablets contain hydroxypropyl methylcellulose, lactose, magnesium stearate, starch, sterotex, and other ingredients. CLINICAL PHARMACOLOGY Propantheline bromide inhibits gastrointestinal motility and diminishes gastric acid secretion. The drug also inhibits the action of acetylcholine at the postganglionic nerve endings of the parasympathetic nervous system. Propantheline bromide is extensively metabolized in man primarily by hydrolysis to the inactive materials xanthene-9-carboxylic acid and (2-hydroxyethyl) diisopropylmethylammonium bromide. In a bioavailability study, peak plasma concentrations of propantheline were achieved in about one hour, following a single oral dose. The plasma elimination half-life of propantheline is about 1.6 hours. Approximately 70% of the dose is excreted in the urine, mostly as metabolites. The urinary excretion of propantheline is about 3% after oral tablet administration. INDICATIONS AND USAGE Propantheline bromide is effective as adjunctive therapy in the treatment of peptic ulcer. CONTRAINDICATIONS Propantheline is contraindicated in patients with: 1. Glaucoma, since mydriasis is to be avoided. 2. Obstructive disease of the gastrointestinal tract (pyloroduodenal stenosis, achalasia, paralytic ileus, etc.). 3. Obstructive uropathy (e.g., bladder-neck obstruction due to prostatic hypertrophy). -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -
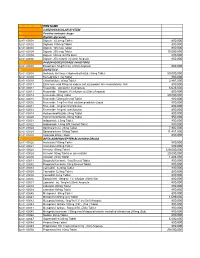
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

Drug Class Review Ophthalmic Cholinergic Agonists
Drug Class Review Ophthalmic Cholinergic Agonists 52:40.20 Miotics Acetylcholine (Miochol-E) Carbachol (Isopto Carbachol; Miostat) Pilocarpine (Isopto Carpine; Pilopine HS) Final Report November 2015 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Carin Steinvoort, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor University of Utah College of Pharmacy Copyright © 2015 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved. Table of Contents Executive Summary ......................................................................................................................... 3 Introduction .................................................................................................................................... 4 Table 1. Glaucoma Therapies ................................................................................................. 5 Table 2. Summary of Agents .................................................................................................. 6 Disease Overview ........................................................................................................................ 8 Table 3. Summary of Current Glaucoma Clinical Practice Guidelines ................................... 9 Pharmacology ............................................................................................................................... 10 Methods ....................................................................................................................................... -

Association Between N-Desmethylclozapine and Clozapine-Induced Sialorrhea
JPET Fast Forward. Published on August 29, 2020 as DOI: 10.1124/jpet.120.000164 This article has not been copyedited and formatted. The final version may differ from this version. 1 Title page Association between N-desmethylclozapine and clozapine-induced sialorrhea: Involvement of increased nocturnal salivary secretion via muscarinic receptors by N- desmethylclozapine Downloaded from Authors and Affiliations: Shuhei Ishikawa, PhD1, 2, a, Masaki Kobayashi, PhD1, 3, *, Naoki Hashimoto, PhD, jpet.aspetjournals.org MD4, Hideaki Mikami, BPharm1, Akihiko Tanimura, PhD5, Katsuya Narumi, PhD1, Ayako Furugen, PhD1, Ichiro Kusumi, PhD, MD4, and Ken Iseki, PhD1 at ASPET Journals on September 23, 2021 1 Laboratory of Clinical Pharmaceutics & Therapeutics, Division of Pharmasciences, Faculty of Pharmaceutical Sciences, Hokkaido University 2 Department of Pharmacy, Hokkaido University Hospital 3 Education Research Center for Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Hokkaido University 4 Department of Psychiatry, Hokkaido University Graduate School of Medicine 5 Department of Pharmacology, School of Dentistry, Health Sciences University of Hokkaido a Present address: Department of Psychiatry, Hokkaido University Hospital JPET Fast Forward. Published on August 29, 2020 as DOI: 10.1124/jpet.120.000164 This article has not been copyedited and formatted. The final version may differ from this version. 2 Running title page Association between N-desmethylclozapine and CIS Corresponding author: Masaki Kobayashi Downloaded from Laboratory of Clinical Pharmaceutics & Therapeutics, Division of Pharmasciences, Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-12-jo, Nishi-6-chome, jpet.aspetjournals.org Kita-ku, Sapporo 060-0812, Japan. Phone/Fax: +81-11-706-3772/3235. E-mail: [email protected] at ASPET Journals on September 23, 2021 Number of text pages: 25 Number of tables: 1 Number of figures: 6 Number of words in the Abstract: 249 Number of words in the Introduction: 686 Number of words in the Discussion: 1492 JPET Fast Forward.