Grindeliae Herba Grindelia 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Sabal May 2017
The Sabal May 2017 Volume 34, number 5 In this issue: Native Plant Project (NPP) Board of Directors May program p1 below Texas at the Edge of the Subtropics— President: Ken King by Bill Carr — p 2-6 Vice Pres: Joe Lee Rubio Native Plant Tour Sat. May 20 in Harlingen — p 7 Secretary: Kathy Sheldon Treasurer: Bert Wessling LRGV Native Plant Sources & Landscapers, Drew Bennie NPP Sponsors, Upcoming Meetings p 7 Ginger Byram Membership Application (cover) p8 Raziel Flores Plant species page #s in the Sabal refer to: Carol Goolsby “Plants of Deep South Texas” (PDST). Sande Martin Jann Miller Eleanor Mosimann Christopher Muñoz Editor: Editorial Advisory Board: Rachel Nagy Christina Mild Mike Heep, Jan Dauphin Ben Nibert <[email protected]> Ken King, Betty Perez Ann Treece Vacek Submissions of relevant Eleanor Mosimann NPP Advisory Board articles and/or photos Dr. Alfred Richardson Mike Heep are welcomed. Ann Vacek Benito Trevino NPP meeting topic/speaker: "Round Table Plant Discussion" —by NPP members and guests Tues., April 23rd, at 7:30pm The Native Plant Project will have a Round Table Plant Discussion in lieu of the usual PowerPoint presentation. We’re encouraging everyone to bring a native plant, either a cutting or in a pot, to be identified and discussed at the meeting. It can be a plant you are unfamiliar with or something that you find remarkable, i.e. blooms for long periods of time or has fruit all winter or is simply gor- geous. We will take one plant at a time and discuss it with the entire group, inviting all comments about your experience with that native. -

Reclassification of North American Haplopappus (Compositae: Astereae) Completed: Rayjacksonia Gen
AmericanJournal of Botany 83(3): 356-370. 1996. RECLASSIFICATION OF NORTH AMERICAN HAPLOPAPPUS (COMPOSITAE: ASTEREAE) COMPLETED: RAYJACKSONIA GEN. NOV.1 MEREDITH A. LANE2 AND RONALD L. HARTMAN R. L. McGregor Herbarium(University of Kansas NaturalHistory Museum Division of Botany) and Departmentof Botany,University of Kansas, Lawrence, Kansas 66047-3729; and Rocky MountainHerbarium, Department of Botany,University of Wyoming,Laramie, Wyoming82071-3165 Rayjacksonia R. L. Hartman& M. A. Lane, gen. nov. (Compositae: Astereae), is named to accommodate the "phyllo- cephalus complex," formerlyof Haplopappus Cass. sect. Blepharodon DC. The new combinationsare R. phyllocephalus (DC.) R. L. Hartman& M. A. Lane, R. annua (Rydb.) R. L. Hartman& M. A. Lane, and R. aurea (A. Gray) R. L. Hartman & M. A. Lane. This transfercompletes the reclassificationof the North American species of Haplopappus sensu Hall, leaving that genus exclusively South American.Rayjacksonia has a base chromosomenumber of x = 6. Furthermore,it shares abruptlyampliate disk corollas, deltatedisk style-branchappendages, and corolla epidermalcell type,among other features,with Grindelia, Isocoma, Olivaea, Prionopsis, Stephanodoria, and Xanthocephalum.Phylogenetic analyses of morphologicaland chloroplastDNA restrictionsite data, taken together,demonstrate that these genera are closely related but distinct. Key words: Astereae; Asteraceae; Compositae; Haplopappus; Rayjacksonia. During the past seven decades, taxonomic application lopappus sensu Hall (1928) are reclassifiedand are cur- -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Report on the Conservation Status of Haplopappus Radiatus, in Idaho
REPORT ON THE CONSERVATION STATUS OF HAPLOPAPPUS RADIATUS, IN IDAHO by Michael Mancuso and Robert K. Moseley Conservation Data Center Nongame and Endangered Wildlife Program June, 1993 Idaho Department of Fish and Game 600 South Walnut, P.O. Box 25 Boise, Idaho 83707 Jerry M. Conley, Director Status Survey Report prepared for Idaho Department of Parks and Recreation through Section 6 funding from U.S. Fish and Wildlife Service, Region 1 REPORT ON THE CONSERVATION STATUS OF i HAPLOPAPPUS RADIATUS, IN IDAHO Taxon Name: Haplopappus radiatus (Nutt.) Cronq. Common Name: Snake River goldenweed Family: Asteraceae (Compositae) States Where Taxon Occurs: U.S.A.; Idaho, Oregon Current Federal Status: Category 1 Candidate Recommended Federal Status: Threatened Authors of Report: Michael Mancuso and Robert K. Moseley Original Date of Report: June 18, 1993 Date of Most Recent Revision: N/A Individual to Whom Further Information and Comments Should be Sent: Robert K. Moseley Conservation Data Center Idaho Dept. Fish and Game P.O. Box 25 Boise, ID 83707 ii ABSTRACT Snake River goldenweed (Haplopappus radiatus) has been recognized as a possible conservation concern for at least twenty years. Until 1991, field inventories in Idaho were limited. In 1991, the Idaho Conservation Data Center completed a field investigation for Snake River goldenweed on the Payette National Forest. Through funding provided by the U.S. Fish and Wildlife Service, additional fieldwork was completed throughout the rest of the species' range in Idaho, during 1992. Survey work has also been done in Oregon. Besides field inventories, research biologists associated with the Conservation Biology Program, Oregon Department of Agriculture, have done cytological and pollination investigations, and presently are monitoring several populations in Oregon. -

Grindelia Squarrosa (Pursh) Dunal - a New Alien Genus and Species for Flora of Georgia
saqarTvelos mecnierebaTa erovnuli akademiis moambe, t. 8, #3, 2014 BULLETIN OF THE GEORGIAN NATIONAL ACADEMY OF SCIENCES, vol. 8, no. 3, 2014 Botany Grindelia squarrosa (Pursh) Dunal - a New Alien Genus and Species for Flora of Georgia Liana Jinjolia, Nana Shakarishvili Institute of Botany, Ilia State University, Tbilisi (Presented by Academy Member Giorgi Nakhutsrishvili) ABSTRACT. Biological invasion is considered as one of the main threats to biodiversity, food security, human health and global economics. Appearance of a new alien species beyond its natural range is always noteworthy because of its possible invasive behavior in a new region. The present paper describes the first occurrence of curlycup gumweed - Grindelia squarrosa (Pursh) Dunal in Georgia. The species is native to North and Central America. The population is represented by perennials occupying an area of about 100 m2 near the village of Karsani (41° 49. 938N, 44° 42.715E, elev. 650 m). Its dense, pure stand demonstrates the ability of sustainable reproduction. Notable signs of the species are conspicuously gummy involucres with phyllaries deflected downward. The acuminate phyllaries are covered with numerous sessile glandular trichomes located in pits. The leaf surfaces are abundantly dotted with sessile multicellular glandular trichomes. The mean length of the awn is 4 ± 0.5 mm; the width in the middle part is ca.108 and ca.65 µm near the tip. Awns are spinulose with the setae of 50 µm length in the middle part and 10 µm near the tip. Polar diameter of the spheroidal pollen grain is 27 - 31 µm. Pollen grains are trisulcate - porate with spinulose exine. -

California Wetlands
VOL. 46, NO.2 FREMONTIA JOURNAL OF THE CALIFORNIA NATIVE PLANT SOCIETY California Wetlands 1 California Native Plant Society CNPS, 2707 K Street, Suite 1; Sacramento, CA 95816-5130 Phone: (916) 447-2677 • Fax: (916) 447-2727 FREMONTIA www.cnps.org • [email protected] VOL. 46, NO. 2, November 2018 Memberships Copyright © 2018 Members receive many benefits, including a subscription toFremontia California Native Plant Society and the CNPS Bulletin. Look for more on inside back cover. ISSN 0092-1793 (print) Mariposa Lily.............................$1,500 Family..............................................$75 ISSN 2572-6870 (online) Benefactor....................................$600 International or library...................$75 Patron............................................$300 Individual................................$45 Gordon Leppig, Editor Plant lover.....................................$100 Student/retired..........................$25 Michael Kauffmann, Editor & Designer Corporate/Organizational 10+ Employees.........................$2,500 4-6 Employees..............................$500 7-10 Employees.........................$1,000 1-3 Employees............................$150 Staff & Contractors Dan Gluesenkamp: Executive Director Elizabeth Kubey: Outreach Coordinator Our mission is to conserve California’s Alfredo Arredondo: Legislative Analyst Sydney Magner: Asst. Vegetation Ecologist native plants and their natural habitats, Christopher Brown: Membership & Sales David Magney: Rare Plant Program Manager and increase understanding, -
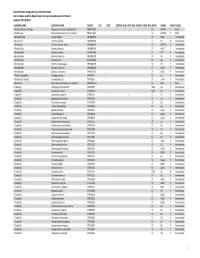
Element Status Designations by Common Name Arizona Game And
Element Status Designations by Common Name Arizona Game and Fish Department, Heritage Data Management System Updated: 10/15/2019 COMMON NAME SCIENTIFIC NAME ELCODE ESA DATE CRITHAB BLM USFS NESL MEXFED SGCN NPL SRANK GRANK TRACK TAXON A Arizona‐Mexican Orange Choisya arizonica var. amplophylla PDRUT02031 S2 G4TNR Y Plant A Balsamroot Balsamorhiza hookeri var. hispidula PDAST11041 S1 G5T3T5 Y Plant A Blueberry Bee Osmia ribifloris IIHYMA2570 S? G4G5 Y Invertebrate A Buckmoth Hemileuca grotei IILEW0M070 S? G4 N Invertebrate A Buckmoth Hemileuca grotei diana IILEW0M072 S? G4T3T4 Y Invertebrate A Bumble Bee Bombus centralis IIHYM24100 S? G4G5 Y Invertebrate A Bumble Bee Bombus fervidus IIHYM24110 S? G4? Y Invertebrate A Bumble Bee Bombus flavifrons IIHYM24120 S? G5 Y Invertebrate A Bumble Bee Bombus huntii IIHYM24140 S? G5 Y Invertebrate A Bumble Bee Bombus melanopygus IIHYM24150 S? G5 Y Invertebrate A Bumble Bee Bombus morrisoni IIHYM24460 S? G4G5 Y Invertebrate A Bumble Bee Bombus nevadensis IIHYM24170 S? G4G5 Y Invertebrate A Bushtail Caddisfly Gumaga griseola IITRI53010 S? G5 Y Invertebrate A Bushtailed Caddisfly Gumaga nigricula IITRI53020 S? G3G4 Y Invertebrate A Buttercup Ranunculus inamoenus var. subaffinis PDRAN0L1C3 S1 G5T1 YPlant A caddisfly Hydropsyche occidentalis IITRI25460 S2S3 G5 Y Invertebrate A caddisfly Hydropsyche oslari IITRIG6010 S2S3 G5 Y Invertebrate A caddisfly Lepidostoma apache IITRI64A10 S S1 G1 Y Invertebrate A Caddisfly Agapetus boulderensis IITRI33190 S? G5 Y Invertebrate A Caddisfly Alisotrichia arizonica IITRID7010 -

Redalyc.Biomass, Resin and Essential Oil Content and Their Variability In
Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas ISSN: 0717-7917 [email protected] Universidad de Santiago de Chile Chile GONZÁLEZ, Benita; VOGEL, Hermine; RAZMILIC, Iván; MARTÍN, José San; DOLL, Ursula Biomass, resin and essential oil content and their variability in natural populations of the Chilean crude drug "bailahuén" (Haplopappus spp.) Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, vol. 11, núm. 1, 2012, pp. 66-73 Universidad de Santiago de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=85622229007 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative © 2012 Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 11 (1): 66 - 73 ISSN 0717 7917 www.blacpma.usach.cl Artículo Original | Original Article Biomass, resin and essential oil content and their variability in natural populations of the Chilean crude drug “bailahuén” (Haplopappus spp.) [Biomasa, contenido de resina y aceite esencial y su variabilidad en poblaciones naturales de las especies de droga cruda chilena “bailahuén” (Haplopappus spp.)] Benita GONZÁLEZ1, Hermine VOGEL1, Iván RAZMILIC2, José San MARTÍN3 & Ursula DOLL4 1 Facultad de Ciencias Agrarias, Universidad de Talca, Talca, Chile. 2 Instituto de Química de Recursos Naturales, Universidad de Talca, Talca, Chile. 3 Instituto de Biología Vegetal y Biotecnología, Universidad de Talca, Talca, Chile. 4 Facultad de Ciencias Forestales, Universidad de Talca, Talca, Chile. Contactos | Contacts: Benita GONZALEZ - E-mail address: [email protected] Abstract Bailahuén (Haplopappus rigidus, Haplopappus baylahuen, Haplopappus multifolius and Haplopappus taeda; Asteraceae) are medicinal shrubs native to the Andes Mountains of Chile widely used to treat hepatic ailments. -

Asteraceae)§ Karin M.Valant-Vetscheraa and Eckhard Wollenweberb,*
Chemodiversity of Exudate Flavonoids in Seven Tribes of Cichorioideae and Asteroideae (Asteraceae)§ Karin M.Valant-Vetscheraa and Eckhard Wollenweberb,* a Department of Plant Systematics and Evolution Ð Comparative and Ecological Phytochemistry, University of Vienna, Rennweg 14, A-1030 Wien, Austria b Institut für Botanik der TU Darmstadt, Schnittspahnstrasse 3, D-64287 Darmstadt, Germany. E-mail: [email protected] * Author for correspondence and reprint requests Z. Naturforsch. 62c, 155Ð163 (2007); received October 26/November 24, 2006 Members of several genera of Asteraceae, belonging to the tribes Mutisieae, Cardueae, Lactuceae (all subfamily Cichorioideae), and of Astereae, Senecioneae, Helenieae and Helian- theae (all subfamily Asteroideae) have been analyzed for chemodiversity of their exudate flavonoid profiles. The majority of structures found were flavones and flavonols, sometimes with 6- and/or 8-substitution, and with a varying degree of oxidation and methylation. Flava- nones were observed in exudates of some genera, and, in some cases, also flavonol- and flavone glycosides were detected. This was mostly the case when exudates were poor both in yield and chemical complexity. Structurally diverse profiles are found particularly within Astereae and Heliantheae. The tribes in the subfamily Cichorioideae exhibited less complex flavonoid profiles. Current results are compared to literature data, and botanical information is included on the studied taxa. Key words: Asteraceae, Exudates, Flavonoids Introduction comparison of accumulation trends in terms of The family of Asteraceae is distributed world- substitution patterns is more indicative for che- wide and comprises 17 tribes, of which Mutisieae, modiversity than single compounds. Cardueae, Lactuceae, Vernonieae, Liabeae, and Earlier, we have shown that some accumulation Arctoteae are grouped within subfamily Cichori- tendencies apparently exist in single tribes (Wol- oideae, whereas Inuleae, Plucheae, Gnaphalieae, lenweber and Valant-Vetschera, 1996). -

Karyotype Traits in Grindelia Squarrosa (Pursh) Dunal (Asteraceae), an Invasive Plant in Romania
Truta et. al.·Silvae Genetica (2012) 61-4/5, 179-186 Karyotype traits in Grindelia squarrosa (Pursh) Dunal (Asteraceae), an invasive plant in Romania By ELENA TRUTA1),*), GABRIELA VOCHITA1), ADRIAN OPREA2) and CULITA SIRBU3) (Received 3rd February 2012) Abstract Europe (Russia, the Ukraine, Republic of Moldova, Esto- The description of the karyotype features and idio- nia, Lithuania, Czech Republic, Belgium, Sweden, gram in Grindelia squarrosa (Pursh) Dunal (Aster- Latvia, Ireland) (SIRBU and OPREA, 2008). Although the aceae), an invasive plant in Romania, are reported here species is most common in the lower elevations of plains for the first time. The diploid chromosome number is and foothills, it was met, too, at 3000 m altitude in Col- 2n = 2x = 12, in agreement with the data published for orado and New Mexico. In Northern Utah, it occurs in the other species of the genus. The karyomorphological Tony Grove Canyon (2000 m) in the Cache National For- data show that the complements of the studied geno- est, also on disturbed ground throughout the valleys and types have small chromosomes (mean chromosome – in the adjacent Wasatch Mountains at elevations of at length is ± SE = 2.56 ± 0.10 µm, and mean length of X – least 2200 m (MCDONOUGH, 1975; WALSH, 1993). Report- haploid complements is ± SE = 15.33 ± 0.69 µm, with a X ed for the first time in the flora of Romania in 1998 range of variability comprised between 12.87–17.51 µm). The karyotypes are made up of six pairs of metacentric (SIRBU and OPREA, 1998), G. squarrosa can be considered and submetacentric chromosomes, with an identical an invasive alien plant in this country. -

Species Lists
Appendix B: Sepcies Lists Appendix B: Species Lists In this appendix: Plants Mammals Birds Pollinators Fish and Mussels Reptiles and Amphibians Plants Scientific Name Common Name Abutilon theophrasti velvetleaf Acalypha ostryifolia pineland threeseed mercury Acalypha rhomboidea common threeseed mercury Acalypha virginica Virginia threeseed mercury Alliaria petiolata garlic mustard Amaranthus tamariscinus tall amaranth Ambrosia artemisifolia annual ragweed Ambrosia trifida great ragweed Ammannia coccinea valley redstem Amorpha brachycarpa leadplant Ampelopsis cordata heartleaf peppervine Amphicarpaea bracteata var. comosa American hogpeanut Amsonia illustris Ozark bluestar Anemone canadensis Canadian anemone Apocynum cannabinum Indian hemp Aristolochia tomentosa Woolly dutchman's pipe Artemisia annua sweet sagewort Asarum canadense Canadian wildginger Asclepias incarnata swamp milkweed Asclepias purpurascens purple milkweed Asclepias syriaca common milkweed Asclepias verticillata whorled milkweed Aster lateriflorus calico aster Aster pilosus hairy white oldfield aster Aster subulatus eastern annual saltmarsh aster Bergia texana Texas bergia Bidens cernua nodding beggerstick Bidens connata purplestem beggarticks Boehmeria cylindrica smallspike false nettle Callitriche terrestris terrestrial water-starwort Calystegia sepium hedge false bindweed Campsis radicans trumpet creeper Cardamine hirsuta hairy bittercress Carex crus-corvi ravenfoot sedge Carex hyalinolepis shoreline sedge, thinscale sedge Carex molesta troublesome sedge Cassia fasciculata -

Vascular Type Specimens
University of Colorado Herbarium (COLO) 1201 Record(s) Page 1 of 322 COLO Type Specimens Acanthaceae Family: Acanthaceae Beloperone fragilis Robinson Type Status: Isotype Accession No: 422655 Bar Code: 351023 Proofed: none. Location: San Luis Potosi, Mexico. Las Canoas. Habitat: Limestone ledges. Collector: C.G. Pringle #3933. Date: 30 October, 5 December 1891. Miscellaneous: Plantae Mexicanae. Gift from Colorado College 1984. Repr. Status: Flr. Annotations: = Beloperone tenera (Rob.) Turrill. References: Proc. Amer. Acad. 27:183. 1892. Beloperone pringlei S. Watson Type Status: Isotype Accession No: 422657 Bar Code: 351031 Proofed: none. Location: Nuevo Leon, Mexico. Hills near Monterey. Habitat: none. Collector: C.G. Pringle #2548. Date: 15 July & 1 August 1889. Miscellaneous: Plantae Mexicanae. Gift from Colorado College 1984. Repr. Status: Flr. Annotations: = Justicia straminea D. Gibson. References: Proc. Amer. Acad. 25:160. 1890. Carlowrightia glandulosa Rob. & Greenm. Type Status: Isotype Accession No: 422658 Bar Code: 356568 Proofed: none. Location: Oaxaca, Mexico. Monte Alban near Oaxaca. Habitat: none. 5500 ft. Collector: C.G. Pringle #6276. Date: 5 December 1895. Miscellaneous: 1-3 feet. Plantae Mexicanae. Gift from Colorado College 1984. Repr. Status: Flr & Frt. Annotations: none. References: Proc. Amer. Acad. 32:40. 1896. Carlowrightia ovata Gray Type Status: Isotype Accession No: 422659 Bar Code: 356576 Proofed: none. Location: Chihuahua, Mexico. Ledges near Chihuahua. Habitat: none. Collector: C.G. Pringle #932. Date: 30 August 1885. Miscellaneous: Plantae Mexicanae. Gift from Colorado College 1984. Repr. Status: Flr & Frt. Annotations: none. References: Proc. Amer. Acad. 21:406. 1886. Page 2 of 322 COLO Type Specimens Acanthaceae Carlowrightia pringlei Rob. & Greenm. Type Status: Isotype Accession No: 422660 Bar Code: 356584 Proofed: none.