Pattern Formation in Nature: Physical Constraints and Self-Organising Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title: Fractals Topics: Symmetry, Patterns in Nature, Biomimicry Related Disciplines: Mathematics, Biology Objectives: A. Learn
Title: Fractals Topics: symmetry, patterns in nature, biomimicry Related Disciplines: mathematics, biology Objectives: A. Learn about how fractals are made. B. Think about the mathematical processes that play out in nature. C. Create a hands-on art project. Lesson: A. Introduction (20 minutes) Fractals are sets in mathematics based on repeated processes at different scales. Why do we care? Fractals are a part of nature, geometry, algebra, and science and are beautiful in their complexity. Fractals come in many types: branching, spiral, algebraic, and geometric. Branching and spiral fractals can be found in nature in many different forms. Branching is seen in trees, lungs, snowflakes, lightning, and rivers. Spirals are visible in natural forms such as hurricanes, shells, liquid motion, galaxies, and most easily visible in plants like flowers, cacti, and Romanesco Figure 1: Romanesco (Figure 1). Branching fractals emerge in a linear fashion. Spirals begin at a point and expand out in a circular motion. A cool demonstration of branching fractals can be found at: http://fractalfoundation.org/resources/what-are-fractals/. Algebraic fractals are based on surprisingly simple equations. Most famous of these equations is the Mandelbrot Set (Figure 2), a plot made from the equation: The most famous of the geometric fractals is the Sierpinski Triangle: As seen in the diagram above, this complex fractal is created by starting with a single triangle, then forming another inside one quarter the size, then three more, each one quarter the size, then 9, 27, 81, all just one quarter the size of the triangle drawn in the previous step. -

On Genes and Form Enrico Coen*, Richard Kennaway and Christopher Whitewoods
© 2017. Published by The Company of Biologists Ltd | Development (2017) 144, 4203-4213 doi:10.1242/dev.151910 REVIEW On genes and form Enrico Coen*, Richard Kennaway and Christopher Whitewoods ABSTRACT aside ‘not because I doubt for a moment the facts nor dispute the The mechanisms by which organisms acquire their sizes and shapes hypotheses nor decry the importance of one or other; but because we through growth was a major focus of D’Arcy Thompson’s book are so much in the dark as to the mysterious field of force in which the On Growth and Form. By applying mathematical and physical chromosomes lie, far from the visible horizon of physical science, principles to a range of biological forms, Thompson achieved fresh that the matter lies (for the present) beyond the range of problems ’ insights, such as the notion that diverse biological shapes could be which this book professes to discuss (p. 341, Thompson, 1942). related through simple deformations of a coordinate system. However, Much of the darkness and mystery Thompson refers to was lifted in Thompson considered genetics to lie outside the scope of his work, the second half of the 20th century, as the nature of genes and their even though genetics was a growing discipline at the time the mechanisms of action became clear. Nevertheless, the link between book was published. Here, we review how recent advances in cell, gene activity and the generation of form remained obscure, largely developmental, evolutionary and computational biology allow because of difficulties in determining growth patterns and relating Thompson’s ideas to be integrated with genes and the processes them to physico-chemical mechanisms. -

John Wells: Centenary Display Jonty Lees: Artist in Residence Autumn 2005 Winter 2007 6 October 2007 – 13 January 2008
Kenneth Martin & Mary Martin: Constructed Works John Wells: Centenary Display Jonty Lees: Artist in Residence Autumn 2005 Winter 2007 6 October 2007 – 13 January 2008 Notes for Teachers - 1 - Contents Introduction 3 Kenneth Martin & Marty Martin: Constructed Works 4 John Wells: Centenary Display 11 Jonty Lees: Artist in Residence 14 Bernard Leach and his Circle 17 Ways of Looking – Questions to Ask of Any Artwork 19 Suggested Activities 20 Tate Resources & Contacts 22 Further Reading 22 Key Art Terms 24 - 2 - Introduction The Winter 2007 displays present: Kenneth Martin and Mary Martin: Constructed Works (Gallery 1, 3, 4, and the Apse) This exhibition shows the work of two of Britain’s key post-war abstract artists, Kenneth Martin and Mary Martin. The exhibition includes nearly 50 works and focuses on Kenneth Martin’s mobiles and his later Chance and Order series of abstract paintings, alongside Mary Martin’s relief sculptures. Modernism and St Ives from 1940 (Lower Gallery 2) This display of artists associated with St Ives from the Tate Collection is designed to complement the Kenneth Martin and Mary Martin exhibition. It includes work by Mary Martin, Victor Pasmore, Anthony Hill and Adrian Heath alongside St Ives artists such as Peter Lanyon, Terry Frost and Ben Nicholson. John Wells: Centenary Display (The Studio) A small display of paintings and relief constructions by John Wells, designed to celebrate the centenary of his birth. Bernard Leach and his Circle (Upper Gallery 2) Ceramics by Bernard Leach and key studio potters who worked alongside him. These works form part of the Wingfield-Digby Collection, recently gifted to Tate St Ives. -

IV. on Growth and Form and D'arcy Wenworth Thompson
Modified 4th chapter from the novel „Problem promatrača“ („Problem of the observer“), by Antonio Šiber, Jesenski i Turk (2008). Uploaded on the author website, http://asiber.ifs.hr IV. On growth and form and D'Arcy Wenworth Thompson St. Andrews on the North Sea is a cursed town for me. In a winter afternoon in year 1941, a bearded, grey-haired eighty year old man with swollen eyes and big head covered by even bigger hat was descending down the hill side to the harbor. He had a huge butcher’s knife in his hand. A few workers that gathered around a carcass of a huge whale which the North Sea washed on the docks visibly retreated away from a disturbing scene of a tall and hairy figure with an impressive knife. The beardo approached the whale carcass and in less than a minute swiftly separated a thick layer of underskin fat, and then also several large pieces of meat. He carefully stored them in a linen bag that he threw over the shoulder and slowly started his return, uphill. The astonished workers watched the whole procedure almost breathlessly, and then it finally occurred to one of them who was looking in the back of a slowly distancing old man that it was not such a bad idea. It is a war time and shortage of meat. And if professor of natural philosophy Sir D’Arcy Thompson concluded that the fish is edible… Who would know better than him? After all, he thinks only of animals and plants. Of course, Thomson knew perfectly well that the whale is not fish, but to workers this was of not much importance. -

Perspectives
PERspECTIVES steady-state spatial patterns could also arise TIMELINE from such processes in living systems21. The full formalization of the nature of Self-organization in cell biology: self-organization processes came from the work of Prigogine on instabilities and the a brief history emergence of organization in ‘dissipative systems’ in the 1960s22–24, and from Haken who worked on similar issues under the Eric Karsenti name of synergetics11 (TIMELINE). Abstract | Over the past two decades, molecular and cell biologists have made It was clear from the outset that the emergence of dynamical organization important progress in characterizing the components and compartments of the observed in physical and chemical systems cell. New visualization methods have also revealed cellular dynamics. This has should be of importance to biology, and raised complex issues about the organization principles that underlie the scientists who are interested in the periodic emergence of coherent dynamical cell shapes and functions. Self-organization manifestations of life and developmental concepts that were first developed in chemistry and physics and then applied to biology have been actively working in this field19,25–29. From a more general point various morphogenetic problems in biology over the past century are now of view, Kauffman built on the ideas of beginning to be applied to the organization of the living cell. Prigogine and Haken in an attempt to explain the origin of order in biology30–32. One of the most fundamental problems in this complex state of living matter as a self- Self-organization was also invoked to biology concerns the origin of forms and organized end8–10. -

Copyrighted Material
1 Symmetry of Shapes in Biology: from D’Arcy Thompson to Morphometrics 1.1. Introduction Any attentive observer of the morphological diversity of the living world quickly becomes convinced of the omnipresence of its multiple symmetries. From unicellular to multicellular organisms, most organic forms present an anatomical or morphological organization that often reflects, with remarkable precision, the expression of geometric principles of symmetry. The bilateral symmetry of lepidopteran wings, the rotational symmetry of starfish and flower corollas, the spiral symmetry of nautilus shells and goat horns, and the translational symmetry of myriapod segments are all eloquent examples (Figure 1.1). Although the harmony that emanates from the symmetry of organic forms has inspired many artists, it has also fascinated generations of biologists wondering about the regulatory principles governing the development of these forms. This is the case for D’Arcy Thompson (1860–1948), for whom the organic expression of symmetries supported his vision of the role of physical forces and mathematical principles in the processes of morphogenesisCOPYRIGHTED and growth. D’Arcy Thompson’s MATERIAL work also foreshadowed the emergence of a science of forms (Gould 1971), one facet of which is a new branch of biometrics, morphometrics, which focuses on the quantitative description of shapes and the statistical analysis of their variations. Over the past two decades, morphometrics has developed a methodological Chapter written by Sylvain GERBER and Yoland SAVRIAMA. 2 Systematics and the Exploration of Life framework for the analysis of symmetry. The study of symmetry is today at the heart of several research programs as an object of study in its own right, or as a property allowing developmental or evolutionary inferences. -

Branching in Nature Jennifer Welborn Amherst Regional Middle School, [email protected]
University of Massachusetts Amherst ScholarWorks@UMass Amherst Patterns Around Us STEM Education Institute 2017 Branching in Nature Jennifer Welborn Amherst Regional Middle School, [email protected] Wayne Kermenski Hawlemont Regional School, [email protected] Follow this and additional works at: https://scholarworks.umass.edu/stem_patterns Part of the Biology Commons, Physics Commons, Science and Mathematics Education Commons, and the Teacher Education and Professional Development Commons Welborn, Jennifer and Kermenski, Wayne, "Branching in Nature" (2017). Patterns Around Us. 2. Retrieved from https://scholarworks.umass.edu/stem_patterns/2 This Article is brought to you for free and open access by the STEM Education Institute at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Patterns Around Us by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. Patterns Around Us: Branching in Nature Teacher Resource Page Part A: Introduction to Branching Massachusetts Frameworks Alignment—The Nature of Science • Overall, the key criterion of science is that it provide a clear, rational, and succinct account of a pattern in nature. This account must be based on data gathering and analysis and other evidence obtained through direct observations or experiments, reflect inferences that are broadly shared and communicated, and be accompanied by a model that offers a naturalistic explanation expressed in conceptual, mathematical, and/or mechanical terms. Materials: -

Art on a Cellular Level Art and Science Educational Resource
Art on a Cellular Level Art and Science Educational Resource Phoenix Airport Museum Educators and Parents, With foundations in art, geometry and plant biology, the objective of this lesson is to recognize patterns and make connections between the inexhaustible variety of life on our planet. This educational resource is geared for interaction with students of all ages to support the understanding between art and science. It has been designed based on our current exhibition, Art on a Cellular Level, on display at Sky Harbor. The questions and activities below were created to promote observation and curiosity. There are no wrong answers. You may print this PDF to use as a workbook or have your student refer to the material online. We encourage educators to expand on this art and science course to create a lesson plan. If you enjoy these activities and would like to investigate further, check back for new projects each week (three projects total). We hope your student will have fun with this and make an art project to share with us. Please send an image of your student’s artwork to [email protected] or hashtag #SkyHarborArts for an opportunity to be featured on Phoenix Sky Harbor International Airport’s social media. Art on a Cellular Level exhibition Sky Harbor, Terminal 4, level 3 Gallery Art is a lens through which we view the world. It can be a tool for storytelling, expressing cultural values and teaching fundamentals of math, technology and science in a visual way. The Terminal 4 gallery exhibition, Art on a Cellular Level, examines the intersections between art and science. -

Theory of the Growth and Evolution of Feather Shape
30JOURNAL R.O. PRUMOF EXPERIMENTAL AND S. WILLIAMSON ZOOLOGY (MOL DEV EVOL) 291:30–57 (2001) Theory of the Growth and Evolution of Feather Shape RICHARD O. PRUM* AND SCOTT WILLIAMSON Department of Ecology and Evolutionary Biology, and Natural History Museum, University of Kansas, Lawrence, Kansas 66045 ABSTRACT We present the first explicit theory of the growth of feather shape, defined as the outline of a pennaceous feather vane. Based on a reanalysis of data from the literature, we pro- pose that the absolute growth rate of the barbs and rachis ridges, not the vertical growth rate, is uniform throughout the follicle. The growth of feathers is simulated with a mathematical model based on six growth parameters: (1) absolute barb and rachis ridge growth rate, (2) angle of heli- cal growth of barb ridges, (3) initial barb ridge number, (4) new barb ridge addition rate, (5) barb ridge diameter, and (6) the angle of barb ramus expansion following emergence from the sheath. The model simulates growth by cell division in the follicle collar and, except for the sixth param- eter, does not account for growth by differentiation in cell size and shape during later keratiniza- tion. The model can simulate a diversity of feather shapes that correspond closely in shape to real feathers, including various contour feathers, asymmetrical feathers, and even emarginate prima- ries. Simulations of feather growth under different parameter values demonstrate that each pa- rameter can have substantial, independent effects on feather shape. Many parameters also have complex and redundant effects on feather shape through their influence on the diameter of the follicle, the barb ridge fusion rate, and the internodal distance. -
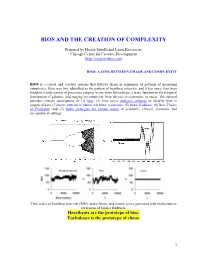
Bios and the Creation of Complexity
BIOS AND THE CREATION OF COMPLEXITY Prepared by Hector Sabelli and Lazar Kovacevic Chicago Center for Creative Development http://creativebios.com BIOS, A LINK BETWEEN CHAOS AND COMPLEXITY BIOS is a causal and creative process that follows chaos in sequences of patterns of increasing complexity. Bios was first identified as the pattern of heartbeat intervals, and it has since then been found in a wide variety of processes ranging in size from Schrodinger’s wave function to the temporal distribution of galaxies, and ranging in complexity from physics to economics to music. This tutorial provides concise descriptions of (1) bios, (2) time series analyses software to identify bios in empirical data, (3) biotic patterns in nature, (4) biotic recursions, (5) biotic feedback, (6) Bios Theory of Evolution, and (7) biotic strategies for human action in scientific, clinical, economic and sociopolitical settings. Time series of heartbeat intervals (RRI), and of biotic and chaotic series generated with mathematical recursions of bipolar feedback. Heartbeats are the prototype of bios. Turbulence is the prototype of chaos. 1 1. BIOS BIOS is an expansive process with chaotic features generated by feedback and characterized by features of creativity. Process: Biotic patterns are sequences of actions or states. Expansive: Biotic patterns continually expand in their diversity and often in their range. This is significant, as natural processes expand, in contrast to convergence to equilibrium, periodic, or chaotic attractors. Expanding processes range from the universe to viruses, and include human populations, empires, ideas and cultures. Chaotic: Biotic series are aperiodic and generated causally; mathematically generated bios is extremely sensitive to initial conditions. -

Word Doc.Corrected Nature As Strategy for Pattern Formation In
Proceedings of Bridges 2015: Mathematics, Music, Art, Architecture, Culture Nature as a Strategy for Pattern Formation in Art Irene Rousseau MFA, Ph.D.,Artist 41Sunset Drive Summit, New Jersey, 07901, USA [email protected], www.irenerousseau.com Abstract We live in a world of structures and patterns that are determined by the interaction of natural forces and environmental factors. Understanding forms in nature provides answers to the molecular structure and how the use of minimal energy creates these patterns. Research by esteemed scientists such as D’Arcy Wentworth Thompson, Charles Darwin and Leonard da Vinci will be noted as evidence for the conclusion. Why is this an important topic? This knowledge can be and has been adapted to many of today’s innovations and uses of technology in medicine and architecture. Applications in these fields will be cited. My creative work will show my strategy for structures and patterns. These are a sequence of numbers combined to stretch the space that show transition and change, that relate to forms in nature. Introduction Where do Structure and Patterns come from? Scientific research has provided the answer, that the molecular structure of a form is determined by intrinsic, natural forces, although random changes are governed by extrinsic, environmental factors of how living organisms evolve and adapt over time. D’Arcy Thompson in his monumental book On Growth and Form states: “Shape or form…is the resultant of a number of forces, which represent or symbolize the manifestations of various kinds of energy…Nature creates structures and patterns according to minimal use of energy”. -

Fractal Art: Closer to Heaven? Modern Mathematics, the Art of Nature, and the Nature of Art
Fractal Art: Closer to Heaven? Modern Mathematics, the art of Nature, and the nature of Art Charalampos Saitis Music & Media Technologies Dept. of Electronic & Electrical Engineering University of Dublin, Trinity College Printing House, Trinity College Dublin 2, Ireland [email protected] Abstract Understanding nature has always been a reference point for both art and science. Aesthetics have put nature at the forefront of artistic achievement. Artworks are expected to represent nature, to work like it. Science has likewise been trying to explain the very laws that determine nature. Technology has provided both sides with the appropriate tools towards their common goal. Fractal art stands right at the heart of the art-science-technology triangle. This paper examines the new perspectives brought to art by fractal geometry and chaos theory and how the study of the fractal character of nature offers promising possibilities towards art’s mission. Introduction “When I judge art, I take my painting and put it next to a God-made object like a tree or flower. If it clashes, it is not art.” Paul Cézanne [1] Mathematics has always been affecting art. Mathematical concepts such as the golden ratio, the platonic solids and the projective geometry have been widely used by painters and sculptors whereas the Pythagorean arithmetic perception of harmony dominates western music to this very day. Infinity and probability theory inspired artists such as M. C. Escher and composers such as Iannis Xenakis forming trends in contemporary art and music. The evolution of technology created new areas of intersection between mathematics and art or music: digital art, computer music and new media.