Complete Dissertation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cult of Isis
Interpreting Early Hellenistic Religion PAPERS AND MONOGRAPHS OF THE FINNISH INSTITUTE AT ATHENS VOL. III Petra Pakkanen INTERPRETING EARL Y HELLENISTIC RELIGION A Study Based on the Mystery Cult of Demeter and the Cult of Isis HELSINKI 1996 © Petra Pakkanen and Suomen Ateenan-instituutin saatiO (Foundation of the Finnish Institute at Athens) 1996 ISSN 1237-2684 ISBN 951-95295-4-3 Printed in Greece by D. Layias - E. Souvatzidakis S.A., Athens 1996 Cover: Portrait of a priest of Isis (middle of the 2nd to middle of the 1st cent. BC). American School of Classical Studies at Athens: Agora Excavations. Inv. no. S333. Photograph Craig Mauzy. Sale: Bookstore Tiedekirja, Kirkkokatu 14, FIN-00170 Helsinki, Finland Contents Acknowledgements I. Introduction 1. Problems 1 2. Cults Studied 2 3. Geographical Confines 3 4. Sources and an Evaluation of Sources 5 11. Methodology 1. Methodological Approach to the History of Religions 13 2. Discussion of Tenninology 19 3. Method for Studying Religious and Social Change 20 Ill. The Cults of Demeter and Isis in Early Hellenistic Athens - Changes in Religion 1. General Overview of the Religious Situation in Athens During the Early Hellenistic Period: Typology of Religious Cults 23 2. Cult of Demeter: Eleusinian Great Mysteries 29 3. Cult of Isis 47 Table 1 64 IV. Problem of the Mysteries 1. Definition of the Tenn 'Mysteries' 65 2. Aspects of the Mysteries 68 3. Mysteries in Athens During the Early Hellenistic Period and a Comparison to Those of Rome in the Third Century AD 71 4. Emergence of the Mysteries ofIsis in Greece 78 Table 2 83 V. -

Annual Report 2014/15
Annual Report 2014/2015 Mission History Colorado inspires generations to find wonder and meaning in our past and to engage in creating a better Colorado. Vision History Colorado leads through accessible, compelling programs in education, preservation, and stewardship; serves Coloradans and enriches communities statewide; connects collections, places, people, and their stories with audiences in meaningful ways; and pursues sustainability through smart planning and sound business practices, while diversifying its financial base. Our Goals To inspire a love of, connection to, and engagement in Colorado and the state’s history To provide excellent stewardship of Colorado’s past through our collections To build an efficient, effective, and financially robust organization to ensure our sustainability into the future All images are from the collections of History Colorado unless otherwise noted. In February 2015, History Colorado Center guests kicked off the opening of The 1968 Exhibit, an award-winning traveling exhibit making stops at some of the nation’s top museums. The 1968 Exhibit brought a pivotal American year to life through photographs, artifacts, vintage pop culture items, and interactives. Letter from the Chair One of the cornerstones of History Colorado’s success has always been, and will continue to be, our partners and supporters. So we’re pleased to present you this report of the highlights and triumphs of our past fiscal year as we continue to inspire generations to find wonder and meaning in our past and to engage in creating a better Colorado. You may have heard about changes at History Colorado’s board, leadership, and staff over this past year. -

Acquisition Research: Creating Synergy for Informed Change
SYM-AM-16-019 Proceedings of the Thirteenth Annual Acquisition Research Symposium Wednesday Sessions Volume I Acquisition Research: Creating Synergy for Informed Change May 4–5, 2016 Published April 30, 2016 Approved for public release; distribution is unlimited. Prepared for the Naval Postgraduate School, Monterey, CA 93943. Acquisition Research Program Graduate School of Business & Public Policy Naval Postgraduate School The research presented in this report was supported by the Acquisition Research Program of the Graduate School of Business & Public Policy at the Naval Postgraduate School. To request defense acquisition research, to become a research sponsor, or to print additional copies of reports, please contact any of the staff listed on the Acquisition Research Program website (www.acquisitionresearch.net). Acquisition Research Program Graduate School of Business & Public Policy Naval Postgraduate School Table of Contents Keynote: The Honorable Sean Stackley, Assistant Secretary of the Navy for Research, Development, & Acquisition ............................................................... vii Plenary Panel: Weapon Acquisition Program Outcomes and Efforts to Reform DoD’s Acquisition Process ..................................................................................... 1 Panel 2. Applications of Real Options Analysis in Defense Acquisition ............ 3 Acquiring Technical Data With Renewable Real Options ....................................... 5 Incorporation of Outcome-Based Contract Requirements in a Real Options -

Perspectives and Theories of Social Innovation for Ageing Population
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Klimczuk, Andrzej; Tomczyk, Łukasz Book — Published Version Perspectives and Theories of Social Innovation for Ageing Population Suggested Citation: Klimczuk, Andrzej; Tomczyk, Łukasz (2020) : Perspectives and Theories of Social Innovation for Ageing Population, Frontiers Media SA, Lausanne, http://dx.doi.org/10.3389/978-2-88963-620-4 This Version is available at: http://hdl.handle.net/10419/226203 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage -

The University of Chicago How to Move a God: Shifting
THE UNIVERSITY OF CHICAGO HOW TO MOVE A GOD: SHIFTING RELIGION AND IMPERIAL IDENTITIES IN ROMAN ATHENS A DISSERTATION SUBMITTED TO THE FACULTY OF THE DIVISION OF THE SOCIAL SCIENCES IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF HISTORY BY JOSHUA RAMON VERA CHICAGO, ILLINOIS JUNE 2018 Copyright © 2018 Joshua Ramon Vera All rights reserved. For Katharyn, άγκυρα µου Contents List of Illustrations vi Acknowledgements vii Abstract xi Introduction: Shifting Landscapes, Shifting Identities 1 Between Tradition and Transition 12 Religious Buildings or Building Religions? 22 Peeling back the Palimpsest 27 Chapter I: A Reputation for Piety 32 The Most God-fearing, as They Say: Classical Athenian Piety 33 The Possession of the Gods: The City as a Sacred Landscape 37 Shrines Made by Human Hands: The Early Christian View 41 Greater than Others in Piety: The Second-Century Perspective 47 The Glory of Your Ancestors: Imagining a Landscape 51 Equal or Opposite Reactions? 56 Whose Landscape Is It Anyway? 65 Chapter II: Memorials to Ancient Virtue 72 Imaging Athens 73 The Emergence of a Core 79 The Classical Image 84 The Heart of the City 95 The Hellenistic Image 103 The Roman Image 107 The Emergence of a Double Core 113 The Hadrianic Image 118 The Mark of the City 123 iv Chapter III: By the People, For the People? 132 Reduced, Reused, or Upcycled? 134 The Road to Recovery 137 Old Money, New Men 140 Roman Plans, Roman Hands? 145 Let Slide the Gods of War 149 A Tale of Two Staircases 161 A Tale of Two Streets 166 Office Space 180 By the -
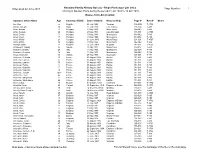
Ships Passenger List Index As of 22 June 2013
Nanaimo Family History Society - Ships Passenger List Index Index as at 22 June 2013 Page Number: 1 Arriving at Quebec Ports during the period 31 Jul 1903 to 13 Oct 1910 Names from Ant to Armo Surname, Given Name Age Country of Birth Date of Arrival Name of Ship Page # Reel # Notes Ant, Mrs. a English 03 July,1908 Tunisian 016-00G T-4759 Antakli, Joseph 24 Syria 21 July,1904 Lake Simcoe 001-00A T-483 Antal, Andras 17 Hungary 11 May,1906 Montezuma 044-042 T-486 Antal, Gergely 26 Hungary 23 May,1910 Lake Michigan 001-001 T-4766 Antal, Gisela 7 Hungary 11 May,1906 Montezuma 044-042 T-486 Antal, Istvan 17 Hungary 11 May,1906 Montezuma 044-042 T-486 Antal, Michal 21 Hungary 01 June,1910 Mount Royal 001-001 T-4767 Antal, Mihaly 32 Hungary 11 May,1906 Montezuma 044-042 T-486 Antaliph, Malke 21 Russian 25 June,1909 Megantic 011-011 T-4761 Antapowicz, Nikolaj 35 Galicia 23 May,1907 Montezuma 007-007 T-489 Antaracci, Donato 24 Italy 11 May,1906 Montezuma 022-020 T-486 Antaracci, Gregoris 16 Italy 11 May,1906 Montezuma 022-020 T-486 Antas, Stanislaw 38 Galicia 27 May,1905 Southwark 001-001 T-484 Antbenac, Bertrand 13 France 03 August,1904 Halifax 011-011 T-483 Antbenac, Carmen 6 France 03 August,1904 Halifax 011-011 T-483 Antbenac, Eugene 50 France 03 August,1904 Halifax 011-011 T-483 Antbenac, Felicia 8 France 03 August,1904 Halifax 011-011 T-483 Antbenac, Gabrielle 7 France 03 August,1904 Halifax 011-011 T-483 Antbenac, Hamilton 10 France 03 August,1904 Halifax 011-011 T-483 Antbenac, Joseph 1y6m France 03 August,1904 Halifax 011-011 T-483 Antbenac, -

Clinical Guidelines
UPDATED 03.01.2019 CLINICAL GUIDELINES Cardiology Services Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt the clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from HealthHelp. All trademarks, product names, logos, and brand names are the property of their respective owners and are used for -

What Are You Eating? Dr
What Are You Eating? Dr. Albert Kausch Food: Big Agriculture, Nutrition, GMOs and Organic Crops Food: Nutrition, GMOs and Organic Crops Myth-Understood The Informed Consumer Questions…. Are there actual risks to our food supply? What are the real issues??? Labeling Safety Where is the evidence? Health New Allergens Gene Flow The StarLink Story The Monarch Butterfly Story Antibiotic Resistant Bacteria Resistance to Pesticides Production of New Toxins Concentration of toxic chemicals and heavy metals Unknown risk and long term harm Although genetically modified organisms (GMOs), primarily plants are used around the world, in food products, as feed and for biofuels, their use has become a contentious issue for some consumers. One issue around the controversy involves proposals regarding the mandatory labeling of food containing ingredients made from GMO crops. Several U.S. states are considering legislation to mandate such labels. © life_edu Supporters of bills to legislate mandatory GMO labeling cite the ‘right to know’ what is in their food. Opponents think that labeling is unnecessary and will only serve to confuse the consumer and raise the costs of food. Further implications extend beyond borders for the consequences of world agriculture concerning this technology © life_edu Hundreds of independent studies have determined that foods made with GMO ingredients are safe and substantially equivalent to their non-GMO counterparts, whether those are grown by conventional or organic agriculture. In addition, hundreds of studies have shown -

TRIBUNE 1960-1969 a - B Last Name First Name Trib
TRIBUNE 1960-1969 A - B Last Name First Name Trib. Date Place Event Aaberg Leslie Merlin 11-Sep-1964 Great Falls Marriage Aafedt Joe 3-Apr-1968 Great Falls Birth Aafedt Joe, Mrs. 1-Feb-1961 Great Falls Birth of son Aafedt Melvin 14-Apr-1961 Great Falls Divorce Aafedt Sharleen K. 14-Apr-1961 Great Falls Divorce Aafedt Sharleen K. from Melvin 18-Feb-1961 Great Falls Divorce Aakre Gilbert 23-Aug-1965 Dutton Obituary Aanerud Adolph A. 27-Aug-1968 Conrad Obituary Aarrestad Abraham C. 13-Sep-1961 Kalispell Obituary Aarrestad Inga 17-Mar-1965 Kalispell Obituary Aarrsted Inga 16-Mar-1965 Kalispell Obituary Aarsvold Christine M. 17-Sep-1966 Great Falls Marriage Aaserud Oswald K., Mrs. 11-Feb-1961 Great Falls Birth of daughter Aaserud Oswald, Mrs. 19-Feb-1964 Great Falls Birth of son Abad Marcus L. 15-Apr-1968 Galen Obituary Abandoned Infant Unknown 9-Feb-1960 Great Falls Death Abare William 2-May-1964 Great Falls Funeral Abare William L. 3-May-1964 Great Falls Funeral Abare William L. 4-May-1964 Great Falls Funeral Abbey Dennis 16-Apr-1968 Great Falls Birth Abbey Woodbury (Mrs.) 13-May-1960 Seattle, WA Obituary Abbey Woodbury (Mrs.) 14-May-1960 Seattle, WA Obituary Abbott Addie Osborne 9-Jul-1966 Lewistown Obituary Abbott Charles A. 13-Jan-1960 Great Falls Birth Abbott Charles A. 26-Dec-1961 Great Falls Birth Abbott Harry 18-Aug-1961 Great Falls Birth Abbott James 23-Apr-1968 Great Falls Birth Abbott James Alfred (Jr.) 8-Nov-1967 Broadus Obituary Abbott Kenneth E. -
Immigrant in America Reel Listing 1
Immigrant in America Reel Listing Aagardd, G. (Gustav), 1852. Belsheim, G. G. Staerke haender. Secret Societies. Minneapolis, Folkebladet. 1900 Mason City, Iowa, [Trinity Lutheran Church]. 1910 Reel: 1, No. 1 Reel: 2, No. 12 Ager, Waldemar, 1869-1941. Beretning om det 18de ordentlige synodemøde. Gamlelandets sønner: fjerde tusen. Decorah, Iowa, Den Norske Synodes Forlag. 1904 Oslo, H. Aschehoug & Co. 1926 Reel: 2, No. 13 Reel: 1, No. 2 Bergh, J. A. (Johan Arndt), 1847. Ager, Waldemar, 1869-1941. Den norsk lutherske kirkes historie i Amerika. I Strømmen. Minneapolis, Minn., the author. 1914 Eau Claire, Wis., Fremad Publishing Co. 1908 printed by Augsburg Publishing House. illustreret af B. Blessum. 2. udg. Reel: 2, No. 14 Reel: 1, No. 3 Bergsland, H. H. Ager, Waldemar, 1869-1941. Gjensvar til pastor Melands "redegjørelse" osv. Kristus for pilatus: en norsk-amerikansk Minnesota, Hauges Synodes Trykkeri. 1895 Fortaelling. Reel: 2, No. 15 Eau Claire, Wis., Fremad Publ. Co. 1910 Reel: 1, No. 4 Biørn, L. M. (Ludvig Marinus), 1835-1908. Pastor P. A. Rasmussen. Alterbog: til brug ved den offentlige Gudstjeneste og Minneapolis, Augsburg Publishing House Trykkeri. de Kirkelige handlinger for Synoden for den 1905 norskev.-luth. kirke i Amerika. Reel: 2, No. 16 Decorah, Iowa, Lutheran Publishing House. 1901 Reel: 1, No. 5 Birkeland, Knut B. (Knut Bergesen), 1857-1925. Brydninger i den Forenede Kirke. Anderson, R. (Rasmus), 1848-1930. Minneapolis, C. Rasmussen's Bogtrykkeri. 1892 Pastor Claus Laurits Clausen. Reel: 2, No. 17 New York, Faas hos Forfatteren, vor Frelsers Danske ev. luth. Kirke. Birkeland, Knut B. (Knut Bergesen), 1857-1925. Kirkehistorisk Bidrag ved R. -

Searching for Authentic Living Through Native Fait
Searching for Authentic Living Through Native Fait Searching for Authentic Living Through Native Fait The Maausk movement in Estonia Jenni Rinne Södertörn University The Library SE-141 89 Huddinge www.sh.se/publications © Jenni Rinne Cover image: untitled, Jenni Rinne Cover layout: Jonathan Robson Graphic form: Per Lindblom & Jonathan Robson Printed by Elanders, Stockholm 2016 Södertörn Doctoral Dissertations 122 ISSN 1652–7399 ISBN 978–91–87843–49–5 (print) ISBN 978–91–87843–50–1 (digital) Abstract The broad aim of this thesis is twofold: firstly, I contextualise the Maausk movement and its practitioners’ understandings in relation to history and the surrounding society; secondly, I analyse the affective and embodied experiences of being a Maausk practitioner from a phenomenological perspective. The thesis focuses on the formation and practice of Maausk, which is perceived to be deeply tied to the society and history where it exists. Relatedly, this study examines how Maausk identity formation and practices have been influenced by the Soviet legacy, romantic nationalism and Estonia’s current economic and political situation. In order to analyse the Maausk experiences and narratives, this study draws from various phenomenologically oriented theories of affect, embodiment and emotion, as well as cultural theories of place, identity, tradition and authenticity. I have used economic anthropology and glo- balisation theories as well as historical studies of Estonia’s Soviet past to contextualise the Maausk movement. Further, to place Maausk in the European religious landscape, this study refers to native faith and Neo- pagan studies. Through sensory ethnography, this study draws on the affective and emotional aspects of the research material to analyse how the complexity of emotional experiences of being a Maausk practitioner produces Maausk meanings and values. -

2018 Annual Report
CASCO, MAINE 2018 ANNUAL REPORT WWW.CAMPSUNSHINE.ORG A RETREAT FOR CHILDREN WITH LIFE-THREATENING ILLNESSES AND THEIR FAMILIES TABLE OF 1 Reflecting on 2018 5 Leading the Way 3 Our Mission 6 First Session CONTENTS 4 Program History 7 Building the Program REFLECTING ON 2018 Another fabulous year is in the books. Thanks to your community service and most importantly was awarded generosity and support our 34th year of serving children Charity Navigator’s coveted top four-star rating for with life-threatening illnesses and their families was a exhibiting fiscal management and transparency. tremendous success! As we look forward to celebrating Each day, into each year we are more astonished by our 35th Anniversary in 2019, we would like to take a the people and organizations that generously share moment and share with you some of the exciting and their time and talent to help insure Camp Sunshine’s amazing highlights of the past year. success. We had people run races, jump in the chilly Camp Sunshine offered 23 programs and served 731 waters, host concerts, produce stage shows, organize families (2,870 family members) from 43 states and 9 golf tournaments, participate in craft fairs and more. countries. We extended our outreach, visiting hospitals Camp hosted our 16th Pumpkin Festival and 2nd annual and clinics in a variety of locations, hosted a pilot Watermelon Festival on the campus of L.L. Bean, program with Boston Children’s Hospital for their Solid Twenty-five brave Navy SEALs paddled 26.2 miles (a Organ Transplant families, partnered with Tropical marathon) atop paddleboards in the open ocean off Smoothie Café to host informational family socials the coast of Coronado Island, CA.