Specialized Impulse Conduction Pathway in the Alligator Heart
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Cardiovascular System 9
Chapter Cardiovascular System 9 Learning Outcomes On completion of this chapter, you will be able to: 1. State the description and primary functions of the organs/structures of the car- diovascular system. 2. Explain the circulation of blood through the chambers of the heart. 3. Identify and locate the commonly used sites for taking a pulse. 4. Explain blood pressure. 5. Recognize terminology included in the ICD-10-CM. 6. Analyze, build, spell, and pronounce medical words. 7. Comprehend the drugs highlighted in this chapter. 8. Describe diagnostic and laboratory tests related to the cardiovascular system. 9. Identify and define selected abbreviations. 10. Apply your acquired knowledge of medical terms by successfully completing the Practical Application exercise. 255 Anatomy and Physiology The cardiovascular (CV) system, also called the circulatory system, circulates blood to all parts of the body by the action of the heart. This process provides the body’s cells with oxygen and nutritive ele- ments and removes waste materials and carbon dioxide. The heart, a muscular pump, is the central organ of the system. It beats approximately 100,000 times each day, pumping roughly 8,000 liters of blood, enough to fill about 8,500 quart-sized milk cartons. Arteries, veins, and capillaries comprise the network of vessels that transport blood (fluid consisting of blood cells and plasma) throughout the body. Blood flows through the heart, to the lungs, back to the heart, and on to the various body parts. Table 9.1 provides an at-a-glance look at the cardiovascular system. Figure 9.1 shows a schematic overview of the cardiovascular system. -

Chapter Xi the Circulatory System and Blood
CHAPTER XI THE CIRCULATORY SYSTEM AND BLOOD Page General characterlstlcs______ __ __ _ __ __ __ __ __ __ _ 239 of these organs are independent of the beating of The pericardium ___ __ __ __ 239 the principal heart, and their primary function is The heart. _____ __ __ 240 Physiology of the heart.______________________________________________ 242 to oscillate the blood within the pallial sinuses. Automatism of heart beat. _ 242 The pacemaker system_ 245 THE PERICARDIUM Methods of study of heart beat_____________________________________ 247 Frequency of beat___ __ __ _ 248 Extracardlac regulatlon____ __ __ _ 250 The heart is located in the pericardium, a thin Effects of mineral salts and drugs___________________________________ 251 Blood vessels_ __ ___ _ 253 walled chamber between the visceral mass and the The arterial system______ __ __ ___ __ __ __ __ __ 253 adductor muscle (fig. 71). In a live oyster the The venous system_________________________________________________ 254 location of the heart is indicated by the throbbing The accessory heart._____________ 258 The blood______ __ __ __ __ __ __ __ 259 of the wall of the pericardium on the left side. Color of blood_ __ __ 261 Here the pericardium wall lies directly under the The hyaline cells___________________________________________________ 261 The granular cells .______________________________________ 262 shell. On the right side the promyal chamber Specific gravity of blood____________________________________________ 265 extends down over the heart region and the mantle Serology ___ __________ __________________ ____ __ ______________________ 265 Bibliography __ __ __ __ __ __ __ 266 separates the pericardium wall from the shell. The cavity in which the heart is lodged is slightly A heart, arteries, veins, and open sinuses form asymmetrical; on the right side it extends farther the circulatory system of oysters and other bi along the anterior part of the adductor muscle valves. -

Abnormalities Caused by Left Bundle Branch Block - Print Article - JAAPA
Marquette University e-Publications@Marquette Physician Assistant Studies Faculty Research and Physician Assistant Studies, Department Publications 12-17-2010 Abnormalities Caused by Left undB le Branch Block James F. Ginter Aurora Cardiovascular Services Patrick Loftis Marquette University, [email protected] Published version. Journal of the American Academy of Physician Assistants, Vol. 23, No. 12 (December 2010). Permalink. © 2010, American Academy of Physician Assistants and Haymarket Media Inc. Useded with permission. Abnormalities caused by left bundle branch block - Print Article - JAAPA http://www.jaapa.com/abnormalities-caused-by-left-bundle-branch-block/... << Return to Abnormalities caused by left bundle branch block James F. Ginter, MPAS, PA-C, Patrick Loftis, PA-C, MPAS, RN December 17 2010 One of the keys to achieving maximal cardiac output is simultaneous contraction of the atria followed by simultaneous contraction of the ventricles. The cardiac conduction system (Figure 1) coordinates the polarization and contraction of the heart chambers. As reviewed in the earlier segment of this department on right bundle branch block (RBBB), the process begins with a stimulus from the sinoatrial (SA) node. The stimulus is then slowed in the atrioventricular (AV) node, allowing complete contraction of the atria. From there, the stimulus proceeds to the His bundle and then to the left and right bundle branches. The bundle branches are responsible for delivering the stimulus to the Purkinje fibers of the left and right ventricles at the same speed, which allows simultaneous contraction of the ventricles. Bundle branch blocks are common disorders of the cardiac conduction system. They can affect the right bundle, the left bundle, or one of its branches (fascicular block), or they may occur in combination. -
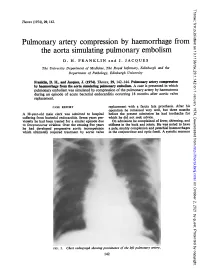
The Aorta Simulating Pulmonary Embolism D
Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. Downloaded from Thorax (1974), 29, 142. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism D. H. FRANKLIN and J. JACQUES The University Department of Medicine, The Royal Infirmary, Edinburgh and the Department of Pathology, Edinburgh University Franklin, D. H., and Jacques, J. (1974). Thorax, 29, 142-144. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism. A case is presented in which pulmonary embolism was simulated by compression of the pulmonary artery by haematoma during an episode of acute bacterial endocarditis occurring 18 months after aortic valve replacement. CASE REPORT replacement with a fascia lata prosthesis. After his operation he remained very well, but three months A 38-year-old male clerk was admitted to hospital before the present admission he had toothache for suffering from bacterial efndocarditis. Seven years pre- which he did not seek advice. viomly he had been treated for a similar episode due On admission he complained of fever, shivering, and to Streptococcus viridans. Over the ensuing five years stiffness in the back and joints. He was noted to have he had developed progressive aortic incompetence a pale, muddy complexion and petechial haemorrhages which ultimately required treatment by aortic valve in the conjunctivae and optic fundi. A systolic murmur http://thorax.bmj.com/ on October 2, 2021 by guest. Protected copyright. FIG. 1. Chesvt radiograph showing prominence of the left pulmonary artery. 142 Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. -

Basic ECG Interpretation
12/2/2016 Basic Cardiac Anatomy Blood Flow Through the Heart 1. Blood enters right atrium via inferior & superior vena cava 2. Right atrium contracts, sending blood through the tricuspid valve and into the right ventricle 3. Right ventricle contracts, sending blood through the pulmonic valve and to the lungs via the pulmonary artery 4. Re-oxygenated blood is returned to the left atrium via the right and left pulmonary veins 5. Left atrium contracts, sending blood through the mitral valve and into the left ventricle 6. Left ventricle contracts, sending blood through the aortic Septum valve and to the body via the aorta 1 http://commons.wikimedia.org/wiki/File:Diagram_of_the_human_heart 2 _(cropped).svg Fun Fact….. Layers of the Heart Pulmonary Artery – The ONLY artery in the body that carries de-oxygenated blood Pulmonary Vein – The ONLY vein in the body that carries oxygenated blood 3 4 Layers of the Heart Endocardium Lines inner cavities of the heart & covers heart valves (Supplies left ventricle) Continuous with the inner lining of blood vessels Purkinje fibers located here; (electrical conduction system) Myocardium Muscular layer – the pump or workhorse of the heart “Time is Muscle” Epicardium Protective outer layer of heart (Supplies SA node Pericardium in most people) Fluid filled sac surrounding heart 5 6 http://stanfordhospital.org/images/greystone/heartCenter/images/ei_0028.gif 1 12/2/2016 What Makes the Heart Pump? Electrical impulses originating in the right atrium stimulate cardiac muscle contraction Your heart's -

Ventricular Anatomy for the Electrophysiologist (Part
Ventricular Anatomy for the REVIEW Electrophysiologist (Part II) SPECIAL 서울대학교 의과대학 병리학교실 서정욱 이화여자대학교 의학전문대학원 김문영 ABSTRACT The conduction fibers and Purkinje network of the ventricular myocardium have their peculiar location and immuno-histochemical characteristics. The bundle of His is located at the inferior border of the membranous septum, where the single trunk ramifies into the left and right bundle branches. The left bundle branches are clearly visible at the surface. The right bundles are hidden in the septal myocardium and it is not easy to recognize them. The cellular characters of the conduction bundles are modified myocardial cells with less cytoplasmic filaments. Myoglobin is expressed at the contractile part, whereas CD56 is expressed at the intercalated disc. A fine meshwork of synaptophysin positive processes is noted particularly at the nodal tissue. C-kit positive cells are scattered, but their role is not well understood. Purkinje cells are a peripheral continuation of bundles seen at the immediate subendocardium of the left ventricle. Key words: ■ conduction system ■ Purkinje network ■ pathology ■ arrhythmia ■ electrophysiology Introduction human heart. In this brief review, the histological characteristics of conduction cells, stained by The functional assessment of abnormal cardiac conventional and immuno-histochemical staining, are 3 rhythm and a targeted treatment based on demonstrated in the second part of the review. electrophysiologic studies are successful advances in cardiology.1 Morphological assessment or confirmation The characteristic location of the ventricular of hearts with such abnormalities is rare, not only due conduction system to the limited availability of human hearts but also inherent technological limitations of existing The atrioventricular node is situated in its technology.2 Classical morphological approaches and subendocardial location at the triangle of Koch. -

Premature Obliteration of the Foramen Ovale by G
PREMATURE OBLITERATION OF THE FORAMEN OVALE BY G. AUSTIN GRESHAM From the Department of Pathology, University of Cambridge Received August 16, 1955 Obliteration of the foramen ovale occurring during intrauterine life is a rare condition. It throws some light on the mechanism of development of endocardial fibroelastosis and also upon the factors concerned in determining the size of the aorta and of the cardiac chambers. CASE HISTORY The patient was the second child of a mother (aet. 21) whose previous obstetric history was normal. Apart from two periods of rather rapid gain of weight for which no cause could be found, the pregnancy was uneventful. The child was eleven days postmature; labour was induced with an enema and lasted forty-five minutes. The infant cried lustily but was cyanosed: the heart was clinically normal. Abnormalities were present in all four limbs. The left radius and ulna were absent and rudimentary digits were present on the skin over the distal end of the limb. Terminal phalanges were absent in the fingers of the right hand, and four toes were present on each foot with a rudimentary fifth digit on the left foot. Cyanosis and dyspniea became more intense and the child died three hours after birth despite the use of oxygen. NECROPSY The body was that of a full-term male infant (weight 3430 g.). The limbs were abnormal as previously described. The lips were blue-black in colour. On the septal wall of the right atrium a hemispherical grey-white area (10x 12 x 4 mm. deep) with a central dimple filled in the usual site of the fossa ovalis (Fig. -

Cardiology Self Learning Package
Cardiology Self Learning Package Module 1: Anatomy and Physiology of the Module 1: Anatomy and Physiology of the Heart Heart. Page 1 Developed by Tony Curran (Clinical Nurse Educator) and Gill Sheppard (Clinical Nurse Specialist) Cardiology (October 2011) CONTENT Introduction…………………………………………………………………………………Page 3 How to use the ECG Self Learning package………………………………………….Page 4 Overview of the Heart…………………………………………………...…………..…….Page 5 Location, Size and Shape of the Heart…………………………………………………Page 5 The Chambers of the Heart…………….………………………………………..……….Page 7 The Circulation System……………………………………….………………..…………Page 8 The Heart Valve Anatomy………………………….…………………………..…………Page 9 Coronary Arteries…………………………………………….……………………..……Page 10 Coronary Veins…………………………………………………………………..……….Page 11 Cardiac Muscle Tissue……………………………………………………………..……Page 12 The Conduction System………………………………………………………………...Page 13 Cardiac Cycle……………………………………………………………………………..Page 15 References…………………………………………………………………………………Page 18 Module Questions………………………………………………………………………..Page 19 Module Evaluation Form………………………………………………………………..Page 22 [Module 1: Anatomy and Physiology of the Heart Page 2 Developed by Tony Curran (Clinical Nurse Educator) and Gill Sheppard (Clinical Nurse Specialist) Cardiology (October 2011) INTRODUCTION Welcome to Module 1: Anatomy and Physiology of the Heart. This self leaning package is designed to as tool to assist nurse in understanding the hearts structure and how the heart works. The goal of this module is to review: Location , size and shape of the heart The chambers of the heart The circulation system of the heart The heart’s valve anatomy Coronary arteries and veins Cardiac muscle tissue The conduction system The cardiac cycle This module will form the foundation of your cardiac knowledge and enable you to understand workings of the heart that will assist you in completing other modules. Learning outcomes form this module are: To state the position of the heart, the size and shape. -

The Cardiac Conduction System in the Rat Expresses the A2 and A3
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 99-103, January 1992 Medical Sciences The cardiac conduction system in the rat expresses the a2 and a3 isoforms of the Na+,K+-ATPase (in situ hybridization/heart conduction system/atrioventricular node/Purkinje strand) RAPHAEL ZAHLER*t, MICHAEL BRINES*, MICHAEL KASHGARIANt, E. J. BENZ, JR.*, AND MAUREEN GILMORE-HEBERT§ Departments of *Internal Medicine, SPathology, and §Therapeutic Radiology, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06510 Communicated by Vincent T. Marchesi, September 5, 1991 ABSTRACT The sodium pump is crucial for the function tially expressed in specialized cardiac conduction tissue. This of the heart and of the cardiac conduction system, which conjecture is reasonable because (i) the cardiac effects of initiates the heartbeat. The a (catalytic) subunit of this pump glycosides are especially prominent in the conduction system has three isoforms; the al isoform is ubiquitous, but the a2 and (although this action is also mediated indirectly via the a3 isoforms are localized to excitable tissue. Because rodent a2 nervous system), (ii) Purkinje fibers contain pumps that are and a3 isoforms are relatively sensitive to ouabain, which also more ouabain-sensitive than those of ventricular muscle, slows cardiac conduction, we studied heart-cell-specific expres- both by electrophysiologic and transport criteria (16-18), (iii) sion of pump isoform genes. Multiple conduction-system struc- electrophysiologic parameters have been found to differ tures, including sinoatrial node, bundle branches, and Pur- between Purkinje myocytes and working ventricular fibers kinje strands, had prominent, specific hybridization signal for (19-22). We thus used the technique of in situ hybridization a2 and a3 isoforms compared with adjacent working myo- to address this issue. -

The Cardiovascular System
11 The Cardiovascular System WHAT The cardiovascular system delivers oxygen and HOW nutrients to the body tissues The heart pumps and carries away wastes blood throughout the body such as carbon dioxide in blood vessels. Blood flow via blood. requires both the pumping action of the heart and changes in blood pressure. WHY If the cardiovascular system cannot perform its functions, wastes build up in tissues. INSTRUCTORS Body organs fail to function properly, New Building Vocabulary and then, once oxygen becomes Coaching Activities for this depleted, they will die. chapter are assignable in hen most people hear the term cardio- only with the interstitial fluid in their immediate Wvascular system, they immediately think vicinity. Thus, some means of changing and of the heart. We have all felt our own “refreshing” these fluids is necessary to renew the heart “pound” from time to time when we are ner- nutrients and prevent pollution caused by vous. The crucial importance of the heart has been the buildup of wastes. Like a bustling factory, the recognized for ages. However, the cardiovascular body must have a transportation system to carry system is much more than just the heart, and its various “cargoes” back and forth. Instead of from a scientific and medical standpoint, it is roads, railway tracks, and subways, the body’s important to understand why this system is so vital delivery routes are its hollow blood vessels. to life. Most simply stated, the major function of the Night and day, minute after minute, our tril- cardiovascular system is transportation. Using lions of cells take up nutrients and excrete wastes. -

The Surgical Treatment of Constrictive Fibrous Endocarditis
The Surgical Treatment of Constrictive Fibrous Endocarditis CH. DUBOST, P. MAURICE, A. GERBAUX, E. BERTRAND, R. RULLIERE, F. VIAL, A. BARRILLON, C. PRIGENT, A. CARPENTIER, R. SOYER Constrictive fibrous endocarditis is a pathological entity described From the Department of Cardiovascular Surgery, by Lo6ffler in 1936. Its etiology is unknown. The clinical course Broussais Hospital, Paris, France is characterized by an evolution towards cardiac insufficiency leading rapidly to a fatal outcome. Modern paraclinical investi- gations are necessary to assess the diagnostic. Cardiac catheteriza- tion brings the proof of adiastole and angiocardiography re- Endocardiectomy was first performed by us in 19715-7 veals the shape of amputation of the ventricle with auriculo- and the patient was improved. Since then, four more cases ventricular regurgitation. The operative procedure consists of have been done, either right or left sided or combined resection of the ventricular fibrosis including the valves and endocardiectomy and there is no doubt that a better auriculo-ventricular valve replacement 'by ;a prosthetic valve. The disease affects both Caucasians and Negros. Our experience knowledge of the clinical aspects of the disease will includes 5 cases. The indications for operation and their results increase the number of cases referred for treatment. are discussed. Pathology C ONSTRICTIVE FIBROUS ENDOCARDITIS, first described The development of fibrous endocarditis is similar in by Loeffler6 in 1936, consists of endocardial fibrosis all cases: it involves the filling chambers of one or several millimeters in thickness which is potentially both ventricles including the papillary muscles and constrictive and limited to the ventricular cavities. chordae tendinae of the mitral and tricuspid valves The clinical course is characterized by an evolution which themselves are included in the process in the towards cardiac insufficiency which may be predomi- majority of cases.8 nantly right or left sided leading rapidly to a fatal outcome. -
The Heart: 2. Subsystems
The Heart – 2., subsystems: Vessels, Nerves, Conduction System & Topography • Coronary arteries • Cardiac veins • Lymphatics • Sympathetic nerves • Parasympaticus • Pacemaking & Conducting system • Topography David Sedmera Charles University First Faculty of Medicine Institute of Anatomy Surface of the Heart Some histology - epicardium A bit of histology - endocardium Some more histology – AV valve Overview of Blood Supply The Blood Supply: Origin Left Right coronary coronary artery artery The Blood Supply: Course The Blood Supply: X-ray - right The Blood Supply: X-ray - left Review: Coronary Arteries and Veins Review: Coronary Arteries and Veins Left coronary artery: Great cardiac vein -anterior interventricular Left oblique atrial vein (of branch Marshall) => coronary sinus --diagonal branch -circumflex branch Middle cardiac vein (with --obtuse marginal branch posterior interventricular branch) Right coronary artery: Small cardiac vein -artery to SA node Anterior cardiac veins (to -acute marginal branch right atrium) -posterior interventricular branch Thebesian veins The Lymphatic Drainage Along the blood vessels The Blood Supply: Troubles... Stenosis of the anterior interventricular ramus of the left coronary artery The Blood Supply: Solution !? PTCA: Percutaneous Transluminar Coronary Arterioplasty Via catheter with balloon The Innervation Parasympathetic: n. X (vagus) - rr. cardiaci Stimulation slows down the rate (S-A node), conduction (A-V node) and decreases force of contraction (via coronary vasoconstriction). Sympathicus comes