FSU ETD Template
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
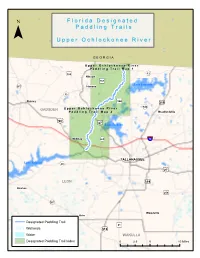
Upper Ochlockonee River Paddling Guide
F ll o r ii d a D e s ii g n a tt e d ¯ P a d d ll ii n g T r a ii ll s U p p e r O c h ll o c k o n e e R ii v e r G E O R G I A U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 1 159 «¬12 )" Hinson )"157 343 Lake Iamonia )" «¬267 Havana «¬12 344 Quincy )" ¤£319 342 GADSDEN U p p e rr O c h ll o c k o n e e R ii v e rr «¬ P a d d ll ii n g T rr a ii ll M a p 2 Bradfordville 90 ¤£ 27 ¤£ Lake Jackson Midway «¬263 ¨¦§10 1)"541 Capitola TALLAHASSEE Lake Talquin «¬20 «¬267 ¤£27 LEON ¤£319 Bloxham )"259 «¬267 Woodville Helen Designated Paddling Trail )"61 Wetlands ¤£319 Water WAKULLA Designated Paddling Trail Index 0 2.5 5 10 Miles 319 ¤£ 61 Newport Arran )" U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 1 ¯ Bell Rd d R d or n c Co o ir a C !| River Ridge «¬12 Plantation Concord Conservation Easement Access Point 1: SR 12 N: 30.6689 W: -84.3051 Havana Hiamonee à Plantation Conservation «¬12 Easement River Ridge Plantation C Conservation o n c Easement o d r R d n a R i id d r e M Kemp Rd N E D Tall Timbers Research Station S D & Land Conservancy A N O G E L Lake Iamonia I ro n B r id g e R d d Pond R ard ch Or !| Mallard Pond Access Point 2: Old Bainbridge Rd Bridge N: 30.5858 W: -84.3594 O l d B Carr Lake a i n b r i d Upper Ochlockonee River Paddling Trail g e R Canoe/Kayak Launch d !| Conservation Lands 27 0 0.5 1 2 Miles ¤£ Wetlands ¯ U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 2 )"270 RCM Farms Conservation Easement O l d B a i -

Florida Historical Uarterly
The Florida Historical uarterly APRIL 1970 PUBLISHED BY THE FLORIDA HISTORICAL SOCIETY FRONT COVER “A View of Pensacola in West Florida” is a black and white engraving published and dedicated by George Gauld to Sir William Burnaby, rear admiral and commander of the British fleet at Jamaica. From the British ensigns on the vessels and the flag flying from the flagstaff, this is obviously a picture of Pensacola during the British period. Since Gauld’s name is not mentioned in any reference sources as an engraver, and since such a skill is not mentioned in his book, it is unlikely that he was the engraver of this picture, but he probably drew the sketch of the scene from which it was made. The engraving is in the Prints and Photographs Division, Library of Congress. Gauld, surveyor of the coasts of Florida, was born in 1732 at Ardbrack, Bamffshire, and he was educated at King’s College, Aberdeen. In 1763 he was appointed to make a survey of all newly acquired English territory in the West Indies, and in March of the following year he sailed aboard the Tartar for Jamaica to join Burnaby’s fleet. In August 1764 he accompanied Sir John Lindsay to Pensacola and he may have made a sketch of the harbor at that time. He was a friend of Philip Pittman, author of The Present State of the European Settlements on the Mississippi . (1770), and Thomas Hutchins whose An Historical Narrative and Topogaphical Description of Louisiana, and West-Florida was published in 1784. They helped him draft charts and plans of West Florida. -

Freshwater Records.Indd
STATE-RECORD FRESHWATER FISH (Information Courtesy of Florida Fish and Wildlife Conservation Commission) Largemouth Bass 17.27 lbs. Billy M. O’Berry July 6,1986 Unnamed lake, Polk County Redeye Bass 7.83 lbs. William T. Johnson Feb. 18, 1989 Apalachicola River, Gadsden Co. Spotted Bass 3.75 lbs. Dow Gilmore June 24, 1985 Apalachicola River, Gulf Co. Suwannee Bass 3.89 lbs. Ronnie Everett March 2,1985 Suwannee River, Gilchrist Co. Striped Bass 42.25 lbs. Alphonso Barnes Dec. 14,1993 Apalachicola River, Gadsden Co. Peacock Bass 9.08 lbs. Jerry Gomez Mar. 11,1993 Kendall Lakes, Dade County Oscar 2.34 lbs. Jimmy Cook Mar. 16,1994 Lake Okeechobee, Palm Beach Skipjack Herring Open (Qualifying weight is 2.5 lbs.) White Bass 4.69 lbs. Richard S. Davis April 9,1982 Apalachicola River, Gadsden Co. Sunshine Bass 16.31 lbs. Thomas R. Elder May 9,1985 Lake Seminole, Jackson County Black Crappie 3.83 lbs. Ben F. Curry, Sr. Jan. 21, 1992 Lake Talquin, Gadsden County Flier 1.24 lbs. William C. Lane, Jr. Aug. 14, 1992 Lake Iamonia, Leon County Bluegill 2.95 lbs. John R. LeMaster Apr. 19,1989 Crystal Lake Washington County Redbreast Sunfish 2.08 lbs. Jerrell DeWees, Jr. April 29, 1988 Suwannee River, Gilchrist County Redear Sunfish 4.86 lbs. Joseph M. Floyd Mar. 13, 1986 Merritts Mill Pond, Jackson Co. Spotted Sunfish .83 lbs. Coy Dotson May 12,1984 Suwannee River, Columbia Co. Warmouth 2.44 lbs. Tony Dempsey Oct. 19, 1985 Yellow Riv. (Guess Lk.) Okaloosa Chain Pickerel 6.96 lbs. -

Sinking Lakes & Sinking Streams in the Wakulla
Nitrogen Contributions of Karst Seepage into the Upper Floridan Aquifer from Sinking Streams and Sinking Lakes in the Wakulla Springshed September 30, 2016 Seán E. McGlynn, Principal Investigator Robert E. Deyle, Project Manager Porter Hole Sink, Lake Jackson (Seán McGlynn, 2000) This project was developed for the Wakulla Springs Alliance by McGlynn Laboratories, Inc. with financial assistance provided by the Fish and Wildlife Foundation of Florida, Inc. through the Protect Florida Springs Tag Grant Program, project PFS #1516-02. Contents Abstract 1 Introduction 2 Data Sources 8 Stream Flow Data 8 Lake Stage, Precipitation, and Evaporation Data 8 Total Nitrogen Concentration Data 10 Data Quality Assurance and Certification 10 Methods for Estimating Total Nitrogen Loadings 11 Precipitation Gains and Evaporation Losses 11 Recharge Factors, Attenuation Factors, and Seepage Rates 11 Findings and Management Recommendations 12 Management Recommendations 17 Recommendations for Further Research 18 References Cited 21 Appendix I: Descriptions of Sinking Waterbodies 23 Sinking Streams (Lotic Systems) 24 Lost Creek and Fisher Creek 26 Black Creek 27 Sinking Lakes (Lentic Systems) 27 Lake Iamonia 27 Lake Munson 28 Lake Miccosukee 28 Lake Jackson 30 Lake Lafayette 31 Bradford Brooks Chain of Lakes 32 Killearn Chain of Lakes 34 References Cited 35 Appendix II: Nitrate, Ammonia, Color, and Chlorophyll 37 Nitrate Loading 38 Ammonia Loading 39 Color Loading 40 Chlorophyll a Loading 41 Abstract This study revises estimates in the 2014 Nitrogen Source Inventory Loading Tool (NSILT) study produced by the Florida Department of Environmental Protection of total nitrogen loadings to Wakulla Springs and the Upper Wakulla River for sinking water bodies based on evaluating flows and water quality data for sinking streams and sinking lakes which were not included in the NSILT. -

2020 Integrated Water Quality Assessment for Florida: Sections 303(D), 305(B), and 314 Report and Listing Update
2020 Integrated Water Quality Assessment for Florida: Sections 303(d), 305(b), and 314 Report and Listing Update Division of Environmental Assessment and Restoration Florida Department of Environmental Protection June 2020 2600 Blair Stone Rd. Tallahassee, FL 32399-2400 floridadep.gov 2020 Integrated Water Quality Assessment for Florida, June 2020 This Page Intentionally Blank. Page 2 of 160 2020 Integrated Water Quality Assessment for Florida, June 2020 Letter to Floridians Ron DeSantis FLORIDA DEPARTMENT OF Governor Jeanette Nuñez Environmental Protection Lt. Governor Bob Martinez Center Noah Valenstein 2600 Blair Stone Road Secretary Tallahassee, FL 32399-2400 June 16, 2020 Dear Floridians: It is with great pleasure that we present to you the 2020 Integrated Water Quality Assessment for Florida. This report meets the Federal Clean Water Act reporting requirements; more importantly, it presents a comprehensive analysis of the quality of our waters. This report would not be possible without the monitoring efforts of organizations throughout the state, including state and local governments, universities, and volunteer groups who agree that our waters are a central part of our state’s culture, heritage, and way of life. In Florida, monitoring efforts at all levels result in substantially more monitoring stations and water quality data than most other states in the nation. These water quality data are used annually for the assessment of waterbody health by means of a comprehensive approach. Hundreds of assessments of individual waterbodies are conducted each year. Additionally, as part of this report, a statewide water quality condition is presented using an unbiased random monitoring design. These efforts allow us to understand the state’s water conditions, make decisions that further enhance our waterways, and focus our efforts on addressing problems. -

Your Guide to Eating Fish Caught in Florida
Fish Consumption Advisories are published periodically by the Your Guide State of Florida to alert consumers about the possibility of chemically contaminated fish in Florida waters. To Eating The advisories are meant to inform the public of potential health risks of specific fish species from specific Fish Caught water bodies. In Florida February 2019 Florida Department of Health Prepared in cooperation with the Florida Department of Environmental Protection and Agriculture and Consumer Services, and the Florida Fish and Wildlife Conservation Commission 2019 Florida Fish Advisories • Table 1: Eating Guidelines for Fresh Water Fish from Florida Waters (based on mercury levels) page 1-50 • Table 2: Eating Guidelines for Marine and Estuarine Fish from Florida Waters (based on mercury levels) page 51-52 • Table 3: Eating Guidelines for species from Florida Waters with Heavy Metals (other than mercury), Dioxin, Pesticides, Polychlorinated biphenyls (PCBs), or Saxitoxin Contamination page 53-54 Eating Fish is an important part of a healthy diet. Rich in vitamins and low in fat, fish contains protein we need for strong bodies. It is also an excellent source of nutrition for proper growth and development. In fact, the American Heart Association recommends that you eat two meals of fish or seafood every week. At the same time, most Florida seafood has low to medium levels of mercury. Depending on the age of the fish, the type of fish, and the condition of the water the fish lives in, the levels of mercury found in fish are different. While mercury in rivers, creeks, ponds, and lakes can build up in some fish to levels that can be harmful, most fish caught in Florida can be eaten without harm. -

Lake Iamonia Habitat Enhancement Leon County, FL
Aquatic Habitat Conservation & Restoration | Florida Fish and Wildlife Conservation Commission Lake Iamonia Habitat Enhancement Leon County, FL Introduction Lake Iamonia, near Tallahassee in the northeast corner of Leon County, is regionally famous for quality sunfish fishing and waterfowl hunting. The 5,757-acre lake exchanges water with the Ochlockonee River during periods of flooding. The majority of the lake’s surface is covered by lily pads and other aquatic vegetation. Lake Iamonia is shallow and contains sinkholes, most of them inactive. Because the lake has a large connected sinkhole, called Iamonia Sink, which drains the lake during severe droughts, natural “drydown” events occurred in 1910, 1917 and 1934. In 1938, an earthen dam was constructed around Iamonia Sink to prevent the lake from disappearing and “save the lake.” This well-intentioned effort prevented the lake from going through natural drought cycles, accelerating the accumulation of organic sediments (muck) on the lake bottom. Although muck development is a natural process, the rapid and excessive accumulation of this material hinders fish reproduction, reduces oxygen levels in the water, fosters floating plant island (tussock) formation and reduces recreational opportunities. Objectives Approach During natural “drydowns,” conduct mechanical removal of excess aquatic plants and associated organic sediments. Muck is most economically removed using conventional heavy equipment on a dry lake bottom. During periods of natural drought, Create additional fish spawning habitat by removing muck such as the Lake Iamonia drydown, the lake water gradually down to the historic mineralized soils. recedes, allowing for most aquatic animals (turtles, fish, and alligators) to seek refuge. A work site is selected near an approved Using mechanical means, reduce the number and volume upland disposal area. -

Your Guide to Eating Fish Caught in Florida
Fish Consumption Advisories are published periodically by the Your Guide State of Florida to alert consumers about the possibility of chemically contaminated fish in Florida waters. To Eating The advisories are meant to inform the public of potential health risks of specific fish species from specific Fish Caught water bodies. In Florida Florida Department of Health Prepared in cooperation with the Florida Department of Environmental Protection and Agriculture and Consumer Services, and the Florida Fish and Wildlife Conservation Commission 2012 Florida Fish Advisories • Table 1: Eating Guidelines for Fresh Water Fish from Florida Waters page 1-29 • Table 2: Eating Guidelines for Marine and Estuarine Fish from Florida Waters page 30-31 • Table 3: Eating Fish from Florida Waters with Dioxin, Pesticide, or Saxitoxin Contamination page 32 Eating Fish is an important part of a healthy diet. Rich in vitamins and low in fat, fish contains protein we need for strong bodies. It is also an excellent source of nutrition for proper growth and development. In fact, the American Heart Association recommends that you eat two meals of fish or seafood every week. At the same time, most Florida seafood has low to medium levels of mercury. Depending on the age of the fish, the type of fish, and the condition of the water the fish lives in, the levels of mercury found in fish are different. While mercury in rivers, creeks, ponds, and lakes can build up in some fish to levels that can be harmful, most fish caught in Florida can be eaten without harm. Florida specific guidelines make eating choices easier. -

Florida Fish and Wildlife Conservation Commission Statewide Alligator Harvest Data Summary
FWC Home : Wildlife & Habitats : Managed Species : Alligator Management Program FLORIDA FISH AND WILDLIFE CONSERVATION COMMISSION STATEWIDE ALLIGATOR HARVEST DATA SUMMARY YEAR AVERAGE LENGTH TOTAL HARVEST FEET INCHES 2000 8 8 2,552 2001 8 8.2 2,268 2002 8 3.7 2,164 2003 8 4.6 2,830 2004 8 5.8 3,237 2005 8 4.9 3,436 2006 8 4.8 6,430 2007 8 6.7 5,942 2008 8 5.1 6,204 2009 8 0 7,844 2010 7 10.9 7,654 2011 8 1.2 8,103 Provisional data 2000 STATEWIDE ALLIGATOR HARVEST DATA SUMMARY AVERAGE LENGTH TOTAL AREA NO AREA NAME FEET INCHES HARVEST 101 LAKE PIERCE 7 9.8 12 102 LAKE MARIAN 9 9.3 30 104 LAKE HATCHINEHA 8 7.9 36 105 KISSIMMEE RIVER (POOL A) 7 6.7 17 106 KISSIMMEE RIVER (POOL C) 8 8.3 17 109 LAKE ISTOKPOGA 8 0.5 116 110 LAKE KISSIMMEE 7 11.5 172 112 TENEROC FMA 8 6.0 1 402 EVERGLADES WMA (WCAs 2A & 2B) 8 8.2 12 404 EVERGLADES WMA (WCAs 3A & 3B) 8 10.4 63 405 HOLEY LAND WMA 9 11.0 2 500 BLUE CYPRESS LAKE 8 5.6 31 501 ST. JOHNS RIVER 1 8 2.2 69 502 ST. JOHNS RIVER 2 8 0.7 152 504 ST. JOHNS RIVER 4 8 3.6 83 505 LAKE HARNEY 7 8.7 65 506 ST. JOHNS RIVER 5 9 2.2 38 508 CRESCENT LAKE 8 9.9 23 510 LAKE JESUP 9 9.5 28 518 LAKE ROUSSEAU 7 9.3 32 520 LAKE TOHOPEKALIGA 9 7.1 47 547 GUANA RIVER WMA 9 4.6 5 548 OCALA WMA 9 8.7 4 549 THREE LAKES WMA 9 9.3 4 601 LAKE OKEECHOBEE (WEST) 8 11.7 448 602 LAKE OKEECHOBEE (NORTH) 9 1.8 163 603 LAKE OKEECHOBEE (EAST) 8 6.8 38 604 LAKE OKEECHOBEE (SOUTH) 8 5.2 323 711 LAKE HANCOCK 9 3.9 101 721 RODMAN RESERVOIR 8 7.0 118 722 ORANGE LAKE 8 9.3 125 723 LOCHLOOSA LAKE 9 3.4 56 734 LAKE SEMINOLE 9 1.5 16 741 LAKE TRAFFORD -

The Ochlockonee River System
Short-eared Owl The Ochlockonee River originates in Worth County, Georgia and flows 190 miles GORDY south and southwest through the Florida Panhandle, where it then empties into the White-tailed Deer Gulf of Mexico at Ochlockonee Bay. BRIDGEBORO OCHLOCKONEE RIVER The Ochlockonee River corridor is home to a wide variety of birds, mammals, reptiles and fish. DOERUN Tributary Network Insect-eating pitcher plants One of the most surprising SIGSBEE grow in bogs along the characteristics of a river system is SALE CITY Ochlockonee. the intricate tributary network that makes up the collecting system. This A River System detail does not show the entire The Watershed A river system is a network of connecting network, only a tiny portion of it. A ridge of high ground borders every river system. channels. Water from rain, snow, groundwa- Even the smallest tributary has its This ridge encloses what is called a watershed. HARTSFIELD SCHLEY ter and other sources collects into the chan- own system of smaller and smaller Beyond the ridge, all water flows into another river nels and flows to the ocean. A river system tributaries until the total number system. Just as water in a bowl flows downward to LANEY FUNSTON has three parts: a collecting system, a trans- becomes astronomical. Most of a common destination, all rivers, creeks, streams, porting system and a dispersing system. the earth’s surface is some type ponds, lakes, wetlands and other types of water MOULTRIE of drainage system. bodies in a watershed drain into the river system. A watershed creates a natural community where HINSONTON every living thing has something in common – the source and final disposition of their water. -

St. Marks River and Apalachee Bay Surface Water Improvement and Management Plan
St. Marks River and Apalachee Bay Surface Water Improvement and Management Plan November 2017 Program Development Series 17-03 Northwest Florida Water Management District St. Marks River and Apalachee Bay Surface Water Improvement and Management Plan November 2017 Program Development Series 17-03 NORTHWEST FLORIDA WATER MANAGEMENT DISTRICT GOVERNING BOARD George Roberts Jerry Pate John Alter Chair, Panama City Vice Chair, Pensacola Secretary-Treasurer, Malone Gus Andrews Jon Costello Marc Dunbar DeFuniak Springs Tallahassee Tallahassee Ted Everett Nick Patronis Bo Spring Chipley Panama City Beach Port St. Joe Brett J. Cyphers Executive Director Headquarters 81 Water Management Drive Havana, Florida 32333-4712 (850) 539-5999 Crestview Econfina Milton 180 E. Redstone Avenue 6418 E. Highway 20 5453 Davisson Road Crestview, Florida 32539 Youngstown, FL 32466 Milton, FL 32583 (850) 683-5044 (850) 722-9919 (850) 626-3101 St. Marks River and Apalachee Bay SWIM Plan Northwest Florida Water Management District Acknowledgements This document was developed by the Northwest Florida Water Management District under the auspices of the Surface Water Improvement and Management (SWIM) Program and in accordance with sections 373.451-459, Florida Statutes. The plan update was prepared under the supervision and oversight of Brett Cyphers, Executive Director and Carlos Herd, Director, Division of Resource Management. Funding support was provided by the National Fish and Wildlife Foundation’s Gulf Environmental Benefit Fund. The assistance and support of the NFWF is gratefully acknowledged. The authors would like to especially recognize members of the public, as well as agency reviewers and staff from the District and from the Ecology and Environment, Inc., team that contributed to the development of this plan. -

Color PDF Version
Jacksonville Rebecca TELFAIR Troy Banks Dawson Louisville Cuthbert BEN HILL JEFF Shellman DAVIS Rutledge TERRELL Fitzgerald PIKE Sasser Leesburg LEE TURNER Goshen Brundidge RANDOLPH Denton BUTLER Luverne Clio BARBOUR Walter F George Reservoir Ashburn Star Muskogee Creek SDAISA 108th CongressColeman of the UnitedLEE States Glenwood Blue Springs Sycamore COFFEE CRENSHAW Fort Gaines CLAY IRWIN Ocilla Ariton Albany Ambrose Brantley Abbeville Edison Morgan Legend Marine Corps Supply Dozier Center (Albany) Sylvester Sumner Bluffton Douglas DISTRICT Tifton Poulan Ty Ty 2 Ozark Arlington CALHOUN Leary DOUGHERTY Putney TIFT DISTRICT Gantt Lake COFFEE Newville 1 Elba DALE Phillipsburg Unionville Enigma Gantt Fort Rucker Military Res MaChis Lower Tama Res Creek SDAISA Lake Tholocco Haleburg WORTH River Baconton Heath Enterprise Fort Headland Alapaha Falls iver Opp Rucker R Lumbee SDAISA New Brockton Midland t Newton Blakely lin Omega Runkle Tactical Site City HENRY BAKER F Sanford BERRIEN KANSAS Willacoochee Babbie Napier Field Newton Doerun OKLAHOMA Andalusia Daleville Grimes Kinsey Pinckard Columbia EARLY Pearson Level Damascus Plains Norman Park ERIE Horn Hill Webb A G Sale City Lenox L E Libertyville Dothan A O Carolina Kinston Clayhatchee B Nashville Onycha R Camilla Featherbed Bay Funston A G COVINGTON Cowarts COLQUITT Sparks Guest Millpond M Coffee Springs Taylor Ashford I Avon A MITCHELL Ellenton Giddens A Colquitt Pond Turley Sweetgum Bay Riverside Moultrie Cherokees of Southeast Arabia Malvern Alabama SDAISA COOK Swamp Samson MILLER HOUSTON