Intermediate Epicotyl Physiological Dormancy in the Recalcitrant Seed of Quercus Chungii with the Elongated Cotyledonary Petiole
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Terminology
PLANT TERMINOLOGY Plant terminology for the identification of plants is a necessary evil in order to be more exact, to cut down on lengthy descriptions, and of course to use the more professional texts. I have tried to keep the terminology in the database fairly simple but there is no choice in using many descriptive terms. The following slides deal with the most commonly used terms (more specialized terms are given in family descriptions where needed). Professional texts vary from fairly friendly to down-right difficult in their use of terminology. Do not be dismayed if a plant or plant part does not seem to fit any given term, or that some terms seem to be vague or have more than one definition – that’s life. In addition this subject has deep historical roots and plant terminology has evolved with the science although some authors have not. There are many texts that define and illustrate plant terminology – I use Plant Identification Terminology, An illustrated Glossary by Harris and Harris (see CREDITS) and others. Most plant books have at least some terms defined. To really begin to appreciate the diversity of plants, a good text on plant systematics or Classification is a necessity. PLANT TERMS - Typical Plant - Introduction [V. Max Brown] Plant Shoot System of Plant – stem, leaves and flowers. This is the photosynthetic part of the plant using CO2 (from the air) and light to produce food which is used by the plant and stored in the Root System. The shoot system is also the reproductive part of the plant forming flowers (highly modified leaves); however some plants also have forms of asexual reproduction The stem is composed of Nodes (points of origin for leaves and branches) and Internodes Root System of Plant – supports the plant, stores food and uptakes water and minerals used in the shoot System PLANT TERMS - Typical Perfect Flower [V. -

The Importance of Petiole Structure on Inhabitability by Ants in Piper Sect. Macrostachys (Piperaceae)
Blackwell Publishing LtdOxford, UKBOJBotanical Journal of the Linnean Society0024-40742007 The Linnean Society of London? 2007 153•• 181191 Original Article ANT DOMATIA IN PIPER SECT. MACROSTACHYS E. J. TEPE ET AL. Botanical Journal of the Linnean Society, 2007, 153, 181–191. With 3 figures The importance of petiole structure on inhabitability by ants in Piper sect. Macrostachys (Piperaceae) ERIC. J. TEPE*, MICHAEL A. VINCENT and LINDA E. WATSON Department of Botany, Miami University, Oxford, OH 45056, USA Received July 2005; accepted for publication August 2006 Several Central American species of Piper sect. Macrostachys have obligate associations with ants, in which the ant partner derives food and shelter from modified plant structures and, in turn, protects the plant against fungal infection and herbivory. In addition to these obligate ant-plants (i.e. myrmecophytes), several other species in Piper have resident ants only sometimes (facultative), and still other plant species never have resident ants. Sheathing petioles of sect. Macrostachys form the domatia in which ants nest. Myrmecophytes in sect. Macrostachys have tightly closed petiole sheaths with bases that clasp the stem. These sheathing petioles appear to be the single most important plant character in the association between ants and species of sect. Macrostachys. We examined the structure and variation of petioles in these species, and our results indicate that minor modifications in a small number of petiolar characters make the difference between petioles that are suitable for habitation by ants and those that are not. © 2007 The Linnean Society of London, Botanical Journal of the Linnean Society, 2007, 153, 181–191. ADDITIONAL KEYWORDS: ant–plant mutualisms – domatia – myrmecophytes – pearl bodies. -

Studies on Seed Germination, Seedling Growth, and in Vitro Shoot
HORTSCIENCE 44(3):751–756. 2009. plantlets are detached from the mother plant that are dried and planted. However, seed propagation is more feasible and recommen- Studies on Seed Germination, Seedling ded for survival of rare species (Van Wyk and Smith, 1996). If this species has to be Growth, and In Vitro Shoot Induction propagated on a large scale by means of seed or tissue culture methods, then currently there of Aloe ferox Mill., a Commercially is no basic information available on these aspects. Aloes are succulent and warm-cli- mate plants, where both temperature and Important Species water play an important role in establishing Michael W. Bairu, Manoj G. Kulkarni, Rene´e A. Street, Rofhiwa them. This study was therefore conducted to B. Mulaudzi, and Johannes Van Staden1 examine 1) the effects of different temper- atures, growth-promoting substances, and Research Centre for Plant Growth and Development, School of Biological watering frequencies on seed germination and Conservation Sciences, University of KwaZulu-Natal Pietermaritzburg, and seedling growth of A. ferox; and 2) to Private Bag X01, Scottsville 3209, South Africa assess the applicability of an in vitro propa- gation protocol developed for other Aloe spp. Additional index words. cytokinins, growth regulators, multiplication rate, smoke solutions, temperature, tissue culture Materials and Methods Abstract. A study was done to investigate the effects of some physical and chemical factors on growth and development of Aloe ferox ex vitro and in vitro. The effects of light, Seed collection. Dried seeds of A. ferox temperature, and smoke–water on seed germination, ex vitro seedling growth require- were collected between the middle to the end ments, and effect of germination medium and cytokinins on shoot induction and of August from the Botanical Garden, Uni- multiplication in vitro were investigated. -

Chapter 5: the Shoot System I: the Stem
Chapter 5 The Shoot System I: The Stem THE FUNCTIONS AND ORGANIZATION OF THE SHOOT SYSTEM PRIMARY GROWTH AND STEM ANATOMY Primary Tissues of Dicot Stems Develop from the Primary Meristems The Distribution of the Primary Vascular Bundles Depends on the Position of Leaves Primary Growth Differs in Monocot and Dicot Stems SECONDARY GROWTH AND THE ANATOMY OF WOOD Secondary Xylem and Phloem Develop from Vascular Cambium Wood Is Composed of Secondary Xylem Gymnosperm Wood Differs from Angiosperm Wood Bark Is Composed of Secondary Phloem and Periderm Buds Are Compressed Branches Waiting to Elongate Some Monocot Stems Have Secondary Growth STEM MODIFICATIONS FOR SPECIAL FUNCTIONS THE ECONOMIC VALUE OF WOODY STEMS SUMMARY ECONOMIC BOTANY: How Do You Make A Barrel? 1 KEY CONCEPTS 1. The shoot system is composed of the stem and its lateral appendages: leaves, buds, and flowers. Leaves are arranged in different patterns (phyllotaxis): alternate, opposite, whorled, and spiral. 2. Stems provide support to the leaves, buds, and flowers. They conduct water and nutrients and produce new cells in meristems (shoot apical meristem, primary and secondary meristems). 3. Dicot stems and monocot stems are usually different. Dicot stems tend to have vascular bundles distributed in a ring, whereas in monocot stems they tend to be scattered. 4. Stems are composed of the following: epidermis, cortex and pith, xylem and phloem, and periderm. 5. Secondary xylem is formed by the division of cells in the vascular cambium and is called wood. The bark is composed of all of the tissues outside the vascular cambium, including the periderm (formed from cork cambium) and the secondary phloem. -
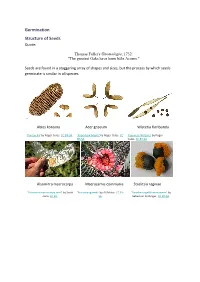
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -

Drupe. Fruit with a Hard Endocarp (Figs. 67 and 71-73); E.G., and Sterculiaceae (Helicteres Guazumaefolia, Sterculia)
Fig. 71. Fig. 72. Fig. 73. Drupe. Fruit with a hard endocarp (figs. 67 and 71-73); e.g., and Sterculiaceae (Helicteres guazumaefolia, Sterculia). Anacardiaceae (Spondias purpurea, S. mombin, Mangifera indi- Desmopsis bibracteata (Annonaceae) has aggregate follicles ca, Tapirira), Caryocaraceae (Caryocar costaricense), Chrysobal- with constrictions between successive seeds, similar to those anaceae (Licania), Euphorbiaceae (Hyeronima), Malpighiaceae found in loments. (Byrsonima crispa), Olacaceae (Minquartia guianensis), Sapin- daceae (Meliccocus bijugatus), and Verbenaceae (Vitex cooperi). Samaracetum. Aggregate of samaras (fig. 74); e.g., Aceraceae (Acer pseudoplatanus), Magnoliaceae (Liriodendron tulipifera Hesperidium. Septicidal berry with a thick pericarp (fig. 67). L.), Sapindaceae (Thouinidium dodecandrum), and Tiliaceae Most of the fruit is derived from glandular trichomes. It is (Goethalsia meiantha). typical of the Rutaceae (Citrus). Multiple Fruits Aggregate Fruits Multiple fruits are found along a single axis and are usually coalescent. The most common types follow: Several types of aggregate fruits exist (fig. 74): Bibacca. Double fused berry; e.g., Lonicera. Achenacetum. Cluster of achenia; e.g., the strawberry (Fra- garia vesca). Sorosis. Fruits usually coalescent on a central axis; they derive from the ovaries of several flowers; e.g., Moraceae (Artocarpus Baccacetum or etaerio. Aggregate of berries; e.g., Annonaceae altilis). (Asimina triloba, Cananga odorata, Uvaria). The berries can be aggregate and syncarpic as in Annona reticulata, A. muricata, Syconium. Syncarp with many achenia in the inner wall of a A. pittieri and other species. hollow receptacle (fig. 74); e.g., Ficus. Drupacetum. Aggregate of druplets; e.g., Bursera simaruba THE GYMNOSPERM FRUIT (Burseraceae). Fertilization stimulates the growth of young gynostrobiles Folliacetum. Aggregate of follicles; e.g., Annonaceae which in species such as Pinus are more than 1 year old. -

Field Identification Guide to WAP Plants 2008 3Rd Ed
The Field Identification Guide to Plants Used in the Wetland Assessment Procedure (WAP) Contributors: Shirley R. Denton, Ph.D. - Biological Research Associates Diane Willis, MS – GPI Southeast, Inc. April 2008 Third Edition (2015 Printing) The Field Identification Guide was prepared by the Southwest Florida Water Management District. Additional copies can be obtained from the District at: Southwest Florida Water Management District Resource Projects Department Ecological Evaluation Section 2379 Broad Street Brooksville, Florida 34604 The Southwest Florida Water Management District (District) does not discriminate on the basis of disability. This nondiscrimination policy involves every aspect of the District’s functions, including access to and participation in the District’s programs and activities. Anyone requiring reasonable accommodation as provided for in the Americans with Disabilities Act should contact the District’s Human Resources Bureau Chief, 2379 Broad St., Brooksville, FL 34604-6899; telephone (352) 796-7211 or 1-800-423-1476 (FL only), ext. 4703; or email [email protected]. If you are hearing or speech impaired, please contact the agency using the Florida Relay Service, 1(800)955-8771 (TDD) or 1(800)955-8770 (Voice). Introduction In 1996, the Florida Legislature directed the Southwest Florida Water Management District (District) to begin the process of establishing Minimum Flows and Levels (MFLs) throughout the District, beginning in Hillsborough, Pasco, and Pinellas counties. MFLs are defined as the flow in watercourses below which significant harm to water resources and ecology of the area would occur, and the level in surface-water bodies and aquifers in which significant harm to the water resources of the area would occur. -

Plant Pathology
Plant Pathology 330-1 Reading / Reference Materials CSU Extension Fact Sheets o Aspen and poplar leaf spots – #2.920 o Backyard orchard: apples and pears [pest management] – #2.800 o Backyard orchard: stone fruits [pest management] – #2.804 o Bacterial wetwood – #2.910 o Cytospora canker – #2.937 o Diseases of roses in Colorado – #2.946 o Dollar spot disease of turfgrass – #2.933 o Dutch elm disease – #5.506 o Dwarf mistletoe management – #2.925 o Fairy ring in turfgrass – #2.908 o Fire blight – #2.907 o Forest fire – Insects and diseases associated with forest fires – #6.309 o Friendly pesticides for home gardens – #2.945 o Greenhouse plant viruses (TSWV-INSV) – #2.947 o Honeylocust diseases – #2.939 o Juniper-hawthorn rust – #2.904 o Juniper-hawthorn rust – #2.904 o Leaf spot and melting out diseases – #2.909 o Necrotic ring spot in turfgrass – #2.900 o Non-chemical disease control – #2.903 o Pesticides – Friendly pesticides for home gardens – #2.945 o Pinyon pine insects and diseases – #2.948 o Powdery mildew – #2.902 o Roses – Diseases of roses in Colorado – #2.946 o Russian olive decline and gummosis – #2.942 o Strawberry diseases – #2.931 o Sycamore anthracnose – #2.930 CSU Extension Publications o Insects and diseases of woody plants of the central Rockies – 506A Curriculum developed by Mary Small, CSU Extension, Jefferson County • Colorado State University, U.S. Department of Agriculture and Colorado counties cooperating. • CSU Extension programs are available to all without discrimination. • No endorsement of products named is intended, nor is criticism implied of products not mentioned. -

Principal Types of Vegetative Shoot Apex Organization in Vascular Plants1
PRINCIPAL TYPES OF VEGETATIVE SHOOT APEX ORGANIZATION IN VASCULAR PLANTS1 RICHARD A. POPHAM Department of Botany and Plant Pathology, The Ohio State University, Columbus 10 Before progress can be made in research, a problem must be recognized. Once the problem has been perceived, a research program may be directed toward a solution. The problem of how and where a shoot grows and the organization of the shoot apex was apparently first conceived by Kaspar Friedrich Wolff (1759). Although his observations on the structure, formation, and growth of cells were fantastically inaccurate, he made a great contribution to our knowledge of the growing plant by setting forth a new and important problem. In a very real sense, Wolff is the father of developmental plant anatomy. Disagreement is the life blood of many research problems. Strenuous opposition is often engendered by a dogmatic statement or a theory which is proposed as a universal truth. Opposition to Wolff's (1759) original proposition regarding the organization and growth of shoot apices prompted plant anatomists, some 85 years later, to investigate the truth of the statement. The factual solution of the problem of shoot apex organization had its beginnings in the work of Nageli (1845). Following this work on many lower cryptogams, Nageli concluded that the cells of all tissues of the shoot of cryptogams and higher plants have their genesis in a single apical cell. The new-born apical cell theory supported by Hofmeister (1851) and others provided the impetus for a renewed, vigorous attack on the problem of shoot apex organization. A little later a new proposal, Hanstein's (1868) histogen theory was born of more careful observations and in a mind unfettered by the prevailing fanaticism of the apical cell theorists. -

Red Shoot Disease of Cranberry
Cranberry Pest Management Red Shoot Disease of Cranberry Red shoot, caused by the fungus Symptoms and spindly with red or yellow Exobasidium perenne, is a rela- leaves that are slightly more tively minor disease of the culti- Signs round than typical oblong cran- vated American cranberry Symptoms Þrst appear in the berry leaves (Figures 1Ð3). By (Vaccinium macrocarpon) and the spring on current yearÕs shoots mid summer the lower surfaces wild small cranberry (Vaccinium that occur singly or as a cluster of leaves become covered with oxycoccus). The red shoot fun- arising from a node on a buried white, powdery fungal spores. gus is related to the fungus that runner. Affected shoots do not Shoots wither after the fungus causes red leaf spot. Red shoot produce ßowers but rather are has shed its spores. Diseased has been reported in Wisconsin, shoots break off easily from the Massachusetts, the PaciÞc Northwest, and in the Atlantic maritime provinces of Canada. Red shoot is not an economi- cally important disease, and speciÞc control recommenda- tions have not been developed. However, the distorted, red or yellow shoots have sometimes been misidentiÞed as a weed, making growers think that an herbicide should be applied. The fact that red shoot is a dis- ease has been conÞrmed by tak- ing fungal spores from infected plants and inoculating them Figure1. Clusters of red shoot in a bed of ‘Searles’. The orangish-rust col- onto healthy plants; the inocu- ored shoots to the left are not affected by red shoot, but rather a different lated plants then developed red problem such as upright dieback. -

Vegetative Vs. Reproductive Morphology
Today’s lecture: plant morphology Vegetative vs. reproductive morphology Vegetative morphology Growth, development, photosynthesis, support Not involved in sexual reproduction Reproductive morphology Sexual reproduction Vegetative morphology: seeds Seed = a dormant young plant in which development is arrested. Cotyledon (seed leaf) = leaf developed at the first node of the embryonic stem; present in the seed prior to germination. Vegetative morphology: roots Water and mineral uptake radicle primary roots stem secondary roots taproot fibrous roots adventitious roots Vegetative morphology: roots Modified roots Symbiosis/parasitism Food storage stem secondary roots Increase nutrient Allow dormancy adventitious roots availability Facilitate vegetative spread Vegetative morphology: stems plumule primary shoot Support, vertical elongation apical bud node internode leaf lateral (axillary) bud lateral shoot stipule Vegetative morphology: stems Vascular tissue = specialized cells transporting water and nutrients Secondary growth = vascular cell division, resulting in increased girth Vegetative morphology: stems Secondary growth = vascular cell division, resulting in increased girth Vegetative morphology: stems Modified stems Asexual (vegetative) reproduction Stolon: above ground Rhizome: below ground Stems elongating laterally, producing adventitious roots and lateral shoots Vegetative morphology: stems Modified stems Food storage Bulb: leaves are storage organs Corm: stem is storage organ Stems not elongating, packed with carbohydrates Vegetative -

Manual of Leaf Architecture
Manual of Leaf Architecture Morphological description and categorization of dicotyledonous and net-veined monocotyledonous angiosperms - 1 - ©1999 by Smithsonian Institution. All rights reserved. Published and distributed by: Leaf Architecture Working Group c/o Scott Wing Department of Paleobiology Smithsonian Institution 10th St. & Constitution Ave., N.W. Washington, DC 20560-0121 ISBN 0-9677554-0-9 Please cite as: Manual of Leaf Architecture - morphological description and categorization of dicotyledonous and net-veined monocotyledonous angiosperms by Leaf Architecture Working Group. 65p. Paper copies of this manual were printed privately in Washington, D.C. We gratefully acknowledge funding from Michael Sternberg and Jan Hartford for the printing of this manual. - 2 - Names and addresses of the Leaf Architecture Working Group in alphabetical order: Amanda Ash Beth Ellis Department of Paleobiology 1276 Cavan St. Smithsonian Institution NHB Boulder, CO 80303 10th St. & Constitution Ave, N.W. Telephone: 303 666-9534 Washington, DC 20560-0121 Email: [email protected] Telephone: 202 357-4030 Fax: 202 786-2832 Email: [email protected] Leo J. Hickey Kirk Johnson Division of Paleobotany Department of Earth and Space Sciences Peabody Museum of Natural History Denver Museum of Natural History Yale University 2001 Colorado Boulevard 170 Whitney Avenue, P.O. Box 208118 Denver, CO 80205-5798 New Haven, CT 06520-8118 Telephone: 303 370-6448 Telephone: 203 432-5006 Fax: 303 331-6492 Fax: 203 432-3134 Email: [email protected] Email: [email protected] Peter Wilf Scott Wing University of Michigan Department of Paleobiology Museum of Paleontology Smithsonian Institution NHB 1109 Geddes Road 10th St. & Constitution Ave, N.W.