Code Short Description Modifier Age Range Rate Effective Date** 10021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lithium Sulfate
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.1 Revision Date 09/14/2012 Print Date 03/12/2014 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Lithium sulfate Product Number : 203653 Brand : Aldrich Supplier : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # (For : (314) 776-6555 both supplier and manufacturer) Preparation Information : Sigma-Aldrich Corporation Product Safety - Americas Region 1-800-521-8956 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Target Organ Effect, Harmful by ingestion. Target Organs Central nervous system, Kidney, Cardiovascular system. GHS Classification Acute toxicity, Oral (Category 4) GHS Label elements, including precautionary statements Pictogram Signal word Warning Hazard statement(s) H302 Harmful if swallowed. Precautionary none statement(s) HMIS Classification Health hazard: 1 Chronic Health Hazard: * Flammability: 0 Physical hazards: 0 NFPA Rating Health hazard: 1 Fire: 0 Reactivity Hazard: 0 Potential Health Effects Inhalation May be harmful if inhaled. May cause respiratory tract irritation. Aldrich - 203653 Page 1 of 7 Skin Harmful if absorbed through skin. May cause skin irritation. Eyes May cause eye irritation. Ingestion Harmful if swallowed. 3. COMPOSITION/INFORMATION ON INGREDIENTS Formula : Li2O4S Molecular Weight : 109.94 g/mol Component Concentration Lithium sulphate CAS-No. 10377-48-7 - EC-No. 233-820-4 4. FIRST AID MEASURES General advice Move out of dangerous area.Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. -

LCD): Computerized Corneal Topography (L33810
Local Coverage Determination (LCD): Computerized Corneal Topography (L33810) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME CONTRACT TYPE CONTRACT NUMBER JURISDICTION STATE(S) First Coast Service Options, Inc. A and B MAC 09102 - MAC B J - N Florida First Coast Service Options, Inc. A and B MAC 09202 - MAC B J - N Puerto Rico First Coast Service Options, Inc. A and B MAC 09302 - MAC B J - N Virgin Islands LCD Information Document Information LCD ID Original Effective Date L33810 For services performed on or after 10/01/2015 LCD Title Revision Effective Date Computerized Corneal Topography For services performed on or after 01/08/2019 Proposed LCD in Comment Period Revision Ending Date N/A N/A Source Proposed LCD Retirement Date N/A N/A AMA CPT / ADA CDT / AHA NUBC Copyright Notice Period Start Date Statement N/A CPT codes, descriptions and other data only are copyright 2019 American Medical Association. All Rights Notice Period End Date Reserved. Applicable FARS/HHSARS apply. N/A Current Dental Terminology © 2019 American Dental Association. All rights reserved. Copyright © 2019, the American Hospital Association, Chicago, Illinois. Reproduced with permission. No portion of the AHA copyrighted materials contained within this publication may be copied without the express written consent of the AHA. AHA copyrighted materials including the UB-04 codes and descriptions may not be removed, copied, or utilized within any Created on 01/02/2020. Page 1 of 7 software, product, service, solution or derivative work without the written consent of the AHA. -

Management and Treatment of Lithium-Induced Nephrogenic Diabetes Insipidus
REVIEW Management and treatment of lithium- induced nephrogenic diabetes insipidus Christopher K Finch†, Lithium carbonate is a well documented cause of nephrogenic diabetes insipidus, with as Tyson WA Brooks, many as 10 to 15% of patients taking lithium developing this condition. Clinicians have Peggy Yam & Kristi W Kelley been well aware of lithium toxicity for many years; however, the treatment of this drug- induced condition has generally been remedied by discontinuation of the medication or a †Author for correspondence Methodist University reduction in dose. For those patients unresponsive to traditional treatment measures, Hospital, Department several pharmacotherapeutic regimens have been documented as being effective for the of Pharmacy, University of management of lithium-induced diabetes insipidus including hydrochlorothiazide, Tennessee, College of Pharmacy, 1265 Union Ave., amiloride, indomethacin, desmopressin and correction of serum lithium levels. Memphis, TN 38104, USA Tel.: +1 901 516 2954 Fax: +1 901 516 8178 [email protected] Lithium carbonate is well known for its wide use associated with a mutation(s) of vasopressin in bipolar disorders due to its mood stabilizing receptors. Acquired causes are tubulointerstitial properties. It is also employed in aggression dis- disease (e.g., sickle cell disease, amyloidosis, orders, post-traumatic stress disorders, conduct obstructive uropathy), electrolyte disorders (e.g., disorders and even as adjunctive therapy in hypokalemia and hypercalcemia), pregnancy, or depression. Lithium has many well documented conditions induced by a drug (e.g., lithium, adverse effects as well as a relatively narrow ther- demeclocycline, amphotericin B and apeutic range of 0.4 to 0.8 mmol/l. Clinically vincristine) [3,4]. Lithium is the most common significant adverse effects include polyuria, mus- cause of drug-induced nephrogenic DI [5]. -

Division of Health Care Financing & Policy SB 278 Section 16
Division of Health Care Financing & Policy SB 278 Section 16 - Physician Rates Reporting Facility & Non-Facility Rate Comparison Nevada 2016 2016 Medicaid Medicaid Medicaid Medicare Medicare vs. vs. Procedure Code & Description Rates (1) Non-Facility Facility Medicare Medicare Rates for Rates for Non-Facility Facility 10021 Fna w/o image 70.92 128.90 72.80 (57.98) (1.88) 10022 Fna w/image 65.39 148.21 68.38 (82.82) (2.99) 10030 Guide cathet fluid drainage 154.73 827.01 176.71 (672.28) (21.98) 10035 Perq dev soft tiss 1st imag 86.35 568.38 90.92 (482.03) (4.57) 10036 Perq dev soft tiss add imag 43.52 495.42 45.82 (451.90) (2.30) 10040 Acne surgery 87.47 106.03 92.10 (18.56) (4.63) 10060 Drainage of skin abscess 95.60 122.68 101.60 (27.08) (6.00) 10061 Drainage of skin abscess 178.04 215.50 188.01 (37.46) (9.97) 10080 Drainage of pilonidal cyst 103.29 188.83 107.87 (85.54) (4.58) 10081 Drainage of pilonidal cyst 172.45 281.57 177.64 (109.12) (5.19) 10120 Remove foreign body 103.22 159.56 108.73 (56.34) (5.51) 10121 Remove foreign body 185.58 287.08 194.44 (101.50) (8.86) 10140 Drainage of hematoma/fluid 118.06 170.87 124.18 (52.81) (6.12) 10160 Puncture drainage of lesion 95.62 136.49 100.72 (40.87) (5.10) 10180 Complex drainage wound 178.97 258.94 188.14 (79.97) (9.17) 11000 Debride infected skin 28.42 56.88 29.76 (28.46) (1.34) 11001 Debride infected skin add-on 14.22 22.40 14.87 (8.18) (0.65) 11004 Debride genitalia & perineum 579.35 608.28 608.28 (28.93) (28.93) 11005 Debride abdom wall 781.65 822.97 822.97 (41.32) (41.32) 11006 Debride -

Current Developments in Corneal Topography and Tomography
diagnostics Review Current Developments in Corneal Topography and Tomography Piotr Kanclerz 1,2,* , Ramin Khoramnia 3 and Xiaogang Wang 4 1 Hygeia Clinic, Department of Ophthalmologyul, Ja´skowaDolina 57, 80-286 Gda´nsk,Poland 2 Helsinki Retina Research Group, University of Helsinki, 00100 Helsinki, Finland 3 The David J. Apple International Laboratory for Ocular Pathology, Department of Ophthalmology, University of Heidelberg, 69120 Heidelberg, Germany; [email protected] 4 Department of Cataract, Shanxi Eye Hospital, Taiyuan 030002, China; [email protected] * Correspondence: [email protected] Abstract: Introduction: Accurate assessment of the corneal shape is important in cataract and refractive surgery, both in screening of candidates as well as for analyzing postoperative outcomes. Although corneal topography and tomography are widely used, it is common that these technologies are confused. The aim of this study was to present the current developments of these technologies and particularly distinguish between corneal topography and tomography. Methods: The PubMed, Web of Science and Embase databases were the main resources used to investigate the medical literature. The following keywords were used in various combinations: cornea, corneal, topography, tomography, Scheimpflug, Pentacam, optical coherence tomography. Results: Topography is the study of the shape of the corneal surface, while tomography allows a three-dimensional section of the cornea to be presented. Corneal topographers can be divided into large- and small-cone Placido-based devices, as well as devices with color-LEDs. For corneal tomography, scanning slit or Scheimpflug imaging and optical coherence tomography may be employed. In several devices, corneal topography and tomography have been successfully combined with tear-film analysis, aberrometry, optical biometry and anterior/posterior segment optical coherence tomography. -

Safety and Effectiveness of the UV-X System for Corneal Collagen Cross
Evaluation of two Riboflavin Dosing Regimens for Corneal Collagen Cross-Linking in Eyes with Progressive Keratoconus or Ectasia Protocol 2010-0243, Version: 15 Protocol Date: September 16, 2016 PHYSICIAN SPONSOR: Francis W. Price, Jr. MD CONFIDENTIAL Background This clinical protocol is designed to evaluate two riboflavin-dosing regimens for treatment of patients with progressive keratoconus or corneal ectasia using investigational technology that increases the cross linking of the corneal stroma using the photochemical interaction of UVA light with the chromophore riboflavin. In this treatment, the corneal stroma is saturated with riboflavin by irrigating the surface after removal of the corneal epithelium. The riboflavin-saturated cornea is then exposed to a uniform field of UVA light with a narrow bandwidth centered at 365 nm. The light is generated by an IROC UV-X irradiation system that creates a uniform 11 mm circle of UVA light. The device has a timer that allows a precise 30-minute exposure of the corneal tissues. The irradiation field of the UVX system produces UVA light with a uniform irradiance of 3 mj/cm2 at the corneal surface. The FDA has classified this technology as a combination product with two components. First is the UV-X light source, which has an LED source and is calibrated and rendered uniform by the use of an optical homogenizer. The second component is a riboflavin ophthalmic solution. This solution is used to saturate the corneal stroma prior to its photochemical activation. This combination product has been studied in a FDA-approved, randomized, placebo- controlled, multi-center trial that has enrolled patients. -
Subchronic Lithium Treatment Increases the Anxiolytic-Like Effect of Mirtazapine on the Expression of Contextual Title Conditioned Fear
Subchronic lithium treatment increases the anxiolytic-like effect of mirtazapine on the expression of contextual Title conditioned fear Author(s) An, Yan; Inoue, Takeshi; Kitaichi, Yuji; Nakagawa, Shin; Wang, Ce; Chen, Chong; Song, Ning; Kusumi, Ichiro European journal of pharmacology, 747, 13-17 Citation https://doi.org/10.1016/j.ejphar.2014.11.009 Issue Date 2015-01-15 Doc URL http://hdl.handle.net/2115/58243 Type article (author version) File Information AnManuscript.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Subchronic lithium treatment increases the anxiolytic-like effect of mirtazapine on the expression of contextual conditioned fear Yan Ana, Takeshi Inouea*, Yuji Kitaichia, Shin Nakagawaa, Ce Wangb, Chong Chena, Ning Songa, Ichiro Kusumia a Department of Psychiatry, Hokkaido University Graduate School of Medicine, North 15, West 7, Kita-ku, Sapporo 060-8638, Japan b Department of Neuropharmacology, Hokkaido University Graduate School of Medicine, North 15, West 7, Kita-ku, Sapporo 060-8638, Japan Number of figures: 3 Corresponding address: Takeshi Inoue, Department of Psychiatry, Hokkaido University Graduate School of Medicine, North 15, West 7, Kita-ku, Sapporo 060-8638, Japan Phone: +81(11)706-5160 Fax: +81(11)706-5081 E-mail: [email protected] 1 Abstract Lithium not only has a mood-stabilizing effect but also the augmentation effect of an antidepressant, the mechanism of which remains unclear. Although lithium may augment the effect of mirtazapine, this augmentation has not been confirmed. Using a contextual fear conditioning test in rats, an animal model of anxiety or fear, we examined the effect of subchronic lithium carbonate (in diet) in combination with systemic mirtazapine on the expression of contextual conditioned fear. -

Refractive Surgery
I explore and get information from inside the eye, that´s my job. You can then go deeper into the diagnosis. You decide, I explore! FOR ACCURACY IN REFRACTIVE SURGERY ACE® is a technology that utilizes the power of high-resolution swept-source OCT imaging to provide the key corneal measurements. Optimizing the quality of the preoperative data provides more information to help you to improve the safety of your refractive surgery procedures.1 ACE® and the TECHNOLAS® TeneoTM 317 Model 2 offer solutions that will refine your results. Transform your daily surgical routine into an exciting day with a platform that brings together corneal topography and tomography and allowing data transfer between both devices. All corneal measurements are based on high-resolution swept-source OCT images KEY FUNCTIONS Corneal topography Corneal wavefront analysis Corneal tomography Differential maps Pachymetry Progression analysis Total corneal power Data transfer with TECHNOLAS® TENEOTM 317 Model 2 * All corneal measurements based on high- resolution swept-source OCT images 1. Muriël Doors et al. Value of optical coherence tomography for anterior segment surgery. J Cataract Refract Surg 2010; 36:1213–1229 Q 2010 ADVANCED CORNEAL EXPLORER HIGHLY CUSTOMIZABLE MAP LAYOUT Display up to 6 maps simultaneously, compare OD and OS, or perform an analysis over time. 12 different map types: Assess each patient’s corneal topography and Anterior and posterior axial or Pachymetry tomography, including tangential curvature Total corneal power curvature and elevation Anterior and posterior elevation maps of the anterior and Anterior and total corneal wavefront (best fit sphere and best fit torus) posterior surfaces. -
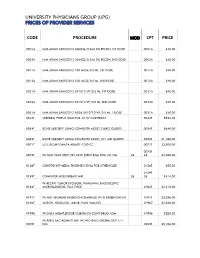
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Comparison of Corneal Topography in Eyes Before and One Month After the Phacoemulsification Procedure
Research Article ISSN: 2574 -1241 DOI: 10.26717/BJSTR.2020.25.004275 Comparison of Corneal Topography in Eyes Before and One Month After the Phacoemulsification Procedure Pateras Evangelos1* and Armenis J2 1Associate Professor, Biomedical Sciences Department, Course Optics & Optometry, University of West Attica, Greece 2Armenis J, Ophthalmologist, MSC candidate, Biomedical Sciences Departmentt, Course Optics & Optometry, University of West Attica, Greece *Corresponding author: Pateras Evangelos, Associate Professor, O O, Biomedical Sciences Department, Course Optics & Optometry, University of West Attica, Greece ARTICLE INFO Abstract Received: February 13, 2020 Antares Placido topographer CSO had been used to measure 50 eyes corneas at Published: February 24, 2020 Ophthalmological Clinic of the Greek Red Cross hospital «Korgialeneio - Benakeio in collaboration with University of West Attica and their refractive maps were taken. The refractive power map was taken before cataract surgery with the method of phaco- Citation: Pateras Evangelos, Armenis J. emulsification. The keratometric measurements for 3 & 5mm were recorded before and Comparison of Corneal Topography in one month after surgery. The correlation of the K1, K2 readings before and one month after the phacoemulsification procedure, do not cause changes in the refractive power of Eyes Before and One Month After the the two main meridians of the cornea of patients, in order to alter the final astigmatism refractive post-operative effect, to a statistically significant degree. J Sci & Tech Res 25(5)-2020. BJSTR. Phacoemulsification Procedure. Biomed Keywords: Cornea; Main Meridians; Astigmatism; With the Rule; Against the Rule; MS.ID.004275. Surgical Incisions; Phacoemulsification; Topography; Correlation Purpose ranging from 2 to 28 µm [11-13]. -

Lithium Sulfate, Technical Grade, Min. 99.0 %
TECHNICAL DATA SHEET Date of Issue: 2018/02/15 Lithium Sulfate, technical grade, min. 99.0 % CAS-No. 10377-48-7 EC-No. 233-820-4 REACH No. 01-2119968668-14 Molecular Formula Li2O4S Product Number 401142 APPLICATION Additive for manufacturing special glass mixtures. Used for marking of chemical products. Additive in the building material industry. SPECIFICATION Li2SO4 min 99.0 % H2O (105 °C) max. 0.4 % Na max. 0.1 % K max. 0.05 % Fe max. 0.002 % Mg max. 0.01 % Heavy metals (as Pb) max. 0.004 % PHYSICAL PROPERTIES Appearance crystalline Color colorless to white Melting point/ range 860 °C Density 2.2 g/cm3 at 20 °C Bulk density ca. 800 kg/m3 Water solubility 342 g/L at 25 °C The information presented herein is believed to be accurate and reliable, but is presented without guarantee or responsibility on the part of Albemarle Corporation and its subsidiaries and affiliates. It is the responsibility of the user to comply with all applicable laws and regulations and to provide for a safe workplace. The user should consider any health or safety hazards or information contained herein only as a guide, and should take those precautions which are necessary or prudent to instruct employees and to develop work practice procedures in order to promote a safe work environment. Further, nothing contained herein shall be taken as an inducement or recommendation to manufacture or use any of the herein materials or processes in violation of existing or future patent. Technical data sheets may change frequently. You can download the latest version from our website www.albemarle-lithium.com. -

Evaluation of Corneal Shape and Biomechanics Before LASIK
Evaluation of Corneal Shape and Biomechanics Before LASIK Renato Ambro´sio, Jr, MD, PhD Leonardo P. Nogueira, MD Diogo L. Caldas, MD Bruno M. Fontes, MD Allan Luz, MD Jorge O. Cazal, MD Milton Ruiz Alves, MD, PhD Michael W. Belin, MD, FACS ’ Introduction The preoperative evaluation is of critical importance for success in laser in situ keratomileusis (LASIK). This examination should fulfill 3 main purposes: counseling and educating the candidates, surgery planning, and screening for cases at higher risk for complications. It is critical to interview each refractive patient to assess their individual needs and to provide realistic expectations. A thorough ophthalmologic examination is mandatory, including specific complementary examina- tions to characterize many aspects of the cornea and the optics of the eye. In fact, it is notable that refractive surgery has motivated tremendous development for advanced diagnostic methods, among many others advancements and innovations in Ophthalmology.1,2 One of the most important aspects of the preoperative examination of LASIK candidates is to screen cases at risk for progressive ectasia. INTERNATIONAL OPHTHALMOLOGY CLINICS Volume 51, Number 2, 11–39 r 2011, Lippincott Williams & Wilkins www.internat-ophthalmology.com | 11 12 ’ Ambro´ sio et al Keratectasia has emerged as a rare but very severe complication of LASIK, which is a leading cause of litigation.3,4 In post-LASIK ectasia, the lamellar cut and excimer laser ablation lead to a state of bio- mechanical failure with an inability to support the continuous stresses caused by intraocular pressure (IOP), extraocular muscle action, blinking, eye rubbing, and other forces.5 The corneal stroma undergoes a 2-step process of delamination and interfibril fracture, which is clinically characterized by thinning and bulging of the cornea.