Effectiveness of Home-Based Fortification of Complementary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State Emergency Commission 2016
January 4, State Emergency Commission 2016 EMERGENCY ASSESSMENT TEAM REPORT ON THE MISSION FOR EVALUATION OF THE WINTER CONDITIONS AND RISK ASSESSMENT Regarding the direction given by the Prime Minister of Mongolia, Emergency Assessment Teams to examine the winter conditions, conduct disaster risk assessments in 21 aimags and solve some urgent issues on the site, established under the Order of the Deputy Prime Minister of Mongolia and Chairperson of the State Emergency Commission, had a mission from 16 December to 28 December 2015 in 21 aimags. The first team headed by Colonel M.Enkh-Amar, a Secretary of the State Emergency Commission, worked in Uvurkhangai, Bayakhongor, Gobi-Altai, Khovd, Bayan-Ulgii, Uvs, Zavkhan, Arkhangai, Khuvsgul, Bulgan, Orkhon, Selenge and Darkhan-Uul aimags. The second team headed by Colonel Ts.Ganzorig, a Deputy Chief of NEMA, worked in Khentii, Dornod, Sukhbaatar, Dornogobi, Umnugobi, Dundgobi, Gobisumber and Tuv aimags. In total, the both teams visited 91 soums of 21 aimags. The teams included the officials and experts from the line ministries and agencies such as State Emergency Commission, Ministry of Food and Agriculture, Ministry of Health and Sports, Ministry of Road and Transport, National Emergency Management Agency, General Police Department, Specialised Inspection Authotiry, Hydro-Meteorological and Environmental Research Centre, and Disaster Research Institute. During the mission, the meetings of the aimag emergency commissions were convened, the situation in the soums with deteriorating winter conditions was examined in the field, herding households were visited, assessment and conclusions were made on the operations of the local branches of the state reserves, thermotransmission lines, the preparedness of power stations and the outreach of herders on remote pasture lands by public and health services, examination of the work for clearing blocked roads and mountain passes was carried out, some issues were solved and necessary assignments and directions were given. -

Fiscal Federalism and Decentralization in Mongolia
Universität Potsdam Ariunaa Lkhagvadorj Fiscal federalism and decentralization in Mongolia Universitätsverlag Potsdam Ariunaa Lkhagvadorj Fiscal federalism and decentralization in Mongolia Ariunaa Lkhagvadorj Fiscal federalism and decentralization in Mongolia Universitätsverlag Potsdam Bibliografische Information der Deutschen Nationalbibliothek Die Deutsche Nationalbibliothek verzeichnet diese Publikation in der Deutschen Nationalbibliografie; detaillierte bibliografische Daten sind im Internet über http://dnb.d-nb.de abrufbar. Universitätsverlag Potsdam 2010 http://info.ub.uni-potsdam.de/verlag.htm Am Neuen Palais 10, 14469 Potsdam Tel.: +49 (0)331 977 4623 / Fax: 3474 E-Mail: [email protected] Das Manuskript ist urheberrechtlich geschützt. Zugl.: Potsdam, Univ., Diss., 2010 Online veröffentlicht auf dem Publikationsserver der Universität Potsdam URL http://pub.ub.uni-potsdam.de/volltexte/2010/4176/ URN urn:nbn:de:kobv:517-opus-41768 http://nbn-resolving.org/urn:nbn:de:kobv:517-opus-41768 Zugleich gedruckt erschienen im Universitätsverlag Potsdam ISBN 978-3-86956-053-3 Abstract Fiscal federalism has been an important topic among public finance theorists in the last four decades. There is a series of arguments that decentralization of governments enhances growth by improving allocation efficiency. However, the empirical studies have shown mixed results for industrialized and developing countries and some of them have demonstrated that there might be a threshold level of economic development below which decentralization is not effective. Developing and transition countries have developed a variety of forms of fiscal decentralization as a possible strategy to achieve effective and efficient governmental structures. A generalized principle of decentralization due to the country specific circumstances does not exist. Therefore, decentra- lization has taken place in different forms in various countries at different times, and even exactly the same extent of decentralization may have had different impacts under different conditions. -
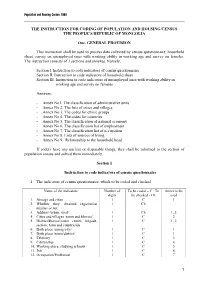
Mongolia 1989 Census Coder
Population and Housing Census 1989 THE INSTRUCTION FOR CODING OF POPULATION AND HOUSING CENSUS THE PEOPLE’S REPUBLIC OF MONGOLIA One. GENERAL PROVISION This instruction shall be used to process data collected by census questionnaire, household sheet, survey on unemployed ones with working ability in working age and survey on females. The instruction consists of 3 sections and annexes. Namely, Section I. Instruction to code indicators of census questionnaire Section II. Instruction to code indicators of household sheet Section III. Instruction to code indicators of unemployed ones with working ability in working age and survey on females Annexes: - Annex No1. The classification of administrative units - Annex No 2. The lists of cities and villages - Annex No 3. The codes for ethnic groups - Annex No 4. The codes for countries - Annex No 5. The classification of national economy - Annex No 6. The classification list of employment - Annex No 7. The classification list of occupation - Annex No 8. Lists of sources of living - Annex No 9. Relationship to the household head If coders have any unclear or disputable things, they shall be informed to the section of population census and solved them immediately. Section I Instruction to code indicators of census questionnaire 1. The indicators of census questionnaire, which to be coded and checked Name of the indicators Number of To be coded – C To Annex to be digits be checked - Ch used 1. Aimags and cities 1 C 1 2. Whether they obtained registration 1 Ch number or not 3. Address /urban, rural/ 1 Ch 1, 2 4. Cities and villages /soum and khoroo/ 1 C 2 5. -

Fiscal Federalism and Decentralization in Mongolia
Munich Personal RePEc Archive Fiscal Federalism and Decentralization in Mongolia Lkhagvadorj, Ariunaa Potsdam University February 2010 Online at https://mpra.ub.uni-muenchen.de/28758/ MPRA Paper No. 28758, posted 17 Feb 2011 10:50 UTC Universität Potsdam Ariunaa Lkhagvadorj Fiscal federalism and decentralization in Mongolia Universitätsverlag Potsdam Ariunaa Lkhagvadorj Fiscal federalism and decentralization in Mongolia Ariunaa Lkhagvadorj Fiscal federalism and decentralization in Mongolia Universitätsverlag Potsdam Bibliografische Information der Deutschen Nationalbibliothek Die Deutsche Nationalbibliothek verzeichnet diese Publikation in der Deutschen Nationalbibliografie; detaillierte bibliografische Daten sind im Internet über http://dnb.d-nb.de abrufbar. Universitätsverlag Potsdam 2010 http://info.ub.uni-potsdam.de/verlag.htm Am Neuen Palais 10, 14469 Potsdam Tel.: +49 (0)331 977 4623 / Fax: 3474 E-Mail: [email protected] Das Manuskript ist urheberrechtlich geschützt. Zugl.: Potsdam, Univ., Diss., 2010 Online veröffentlicht auf dem Publikationsserver der Universität Potsdam URL http://pub.ub.uni-potsdam.de/volltexte/2010/4176/ URN urn:nbn:de:kobv:517-opus-41768 http://nbn-resolving.org/urn:nbn:de:kobv:517-opus-41768 Zugleich gedruckt erschienen im Universitätsverlag Potsdam ISBN 978-3-86956-053-3 Abstract Fiscal federalism has been an important topic among public finance theorists in the last four decades. There is a series of arguments that decentralization of governments enhances growth by improving allocation efficiency. However, the empirical studies have shown mixed results for industrialized and developing countries and some of them have demonstrated that there might be a threshold level of economic development below which decentralization is not effective. Developing and transition countries have developed a variety of forms of fiscal decentralization as a possible strategy to achieve effective and efficient governmental structures. -

Technical Evaluation Report
DRAFT Mongolia Ministry of Finance, Mongolia Ref:WB/MOF/MINIS/CS/QCBS/1.1.4 (c)/2015 Project Name: Mining Infrastructure Investment Support Project (MINIS) Credit# 4888-MN Terms of Reference for the Feasibility Study for “Shuren hydropower plant” project Funded by: International Development Association Date: October2015 1 Terms of Reference for a Feasibility Study of DRAFT ‗Shuren Hydropower Plant‘ Project 2 Terms of Reference for a Feasibility Study of DRAFT ‗Shuren Hydropower Plant‘ Project CONTENTS A. GENERAL INFORMATION ..................................................................................................... 6 A 1. Introduction ..................................................................................................................... 6 A 1.1. Background .............................................................................................................. 6 A 1.2. The Government Policy of Mongolia ....................................................................... 7 A 1.3 Project Region .......................................................................................................... 8 A 1.4. Project initial screening ........................................................................................... 9 A 2. Overall Scope of the Study .............................................................................................. 9 A 2.1. The Purpose of Feasibility Study .............................................................................. 9 A 2.2. Integration with the ESIA ...................................................................................... -

Total: Current Account of Individuals As of December 31, 2020 Million MNT
Total: Current account of individuals As of December 31, 2020 million MNT Current account Number Outstanding No. Aimag, city Soum, district Total Account Owner Male Female Male Female Male Female 1 Total 1,561,111.9 864,298.7 696,813.2 2,503,141 2,969,794 2,786,562 2,779,985 1.1 Western region 90,431.4 50,766.1 39,665.3 226,780 259,832 208,541 236,316 1.1.1 Toal 17,642.5 10,112.2 7,530.4 40,382 45,787 36,828 41,328 1.1.1.1 Uliastai 6,375.3 3,413.9 2,961.5 11,158 14,102 10,623 13,271 1.1.1.2 Aldarkhaan 578.8 370.3 208.5 1,240 1,389 1,083 1,181 1.1.1.3 Asgat 134.2 81.6 52.6 479 532 380 440 1.1.1.4 Bayantes 385.4 202.2 183.2 1,322 1,542 1,109 1,293 1.1.1.5 Bayankhairkhan 427.2 265.0 162.1 947 947 884 858 1.1.1.6 Durvuljin 326.7 191.0 135.7 1,034 1,107 920 947 1.1.1.7 Zavkhanmandal 224.5 144.0 80.4 672 729 559 628 1.1.1.8 Ider 418.1 241.6 176.5 1,337 1,370 1,199 1,206 1.1.1.9 Ikh-Uul 1,053.4 618.7 434.6 2,731 3,026 2,447 2,683 1.1.1.10 Numrug 342.3 214.4 127.9 1,073 1,056 973 958 1.1.1.11 Otgon 536.7 317.5 219.3 1,386 1,416 1,241 1,235 1.1.1.12 Zavkhan Santmargaz 324.1 201.4 122.8 943 1,049 823 899 1.1.1.13 Songino 435.9 278.0 157.9 958 1,023 848 890 1.1.1.14 Tosontsengel 2,051.5 1,124.4 927.1 5,468 6,236 5,124 5,763 1.1.1.15 Tudevtei 321.3 198.9 122.4 1,098 1,135 955 984 1.1.1.16 Telmen 589.1 357.8 231.3 1,395 1,475 1,267 1,352 1.1.1.17 Tes 585.2 389.4 195.8 1,533 1,608 1,370 1,425 1.1.1.18 Urgamal 189.2 93.2 96.1 687 811 604 698 1.1.1.19 Tsagaankhairkhan 308.0 168.9 139.2 737 838 662 733 1.1.1.20 Tsagaanchuluut 371.4 213.3 158.2 717 779 -

Ìîíãîë Íóòàã Äàõü Ò¯¯Õ, Ñî¨Ëûí ¯Ë Õªäëªõ Äóðñãàë
ÃÎÂÜѯÌÁÝÐ ÀÉÌÃÈÉÍ ÍÓÒÀà ÄÀÕÜ Ò¯¯Õ, ÑΨËÛÍ ¯Ë ÕªÄËªÕ ÄÓÐÑÃÀË ISBN 978-99929-61-98-8 Ñî¨ëûí ªâèéí òªâ ÌÎÍÃÎË ÍÓÒÀà ÄÀÕÜ Ò¯¯Õ, ÑΨËÛÍ ¯Ë ÕªÄËªÕ ÄÓÐÑÃÀË Historical and cultural immovable ProPerties in monGolia YII ÄÝâòÝÐ ÃîâÜѯÌÁÝÐ, ÄÀÐÕÀí-ÓÓë, îÐÕîí ÀéÌÀà 1 ÃÎÂÜѯÌÁÝÐ ÀÉÌÃÈÉÍ ÍÓÒÀà ÄÀÕÜ Ò¯¯Õ, ÑΨËÛÍ ¯Ë ÕªÄËªÕ ÄÓÐÑÃÀË ÌÎíãÎë íóòàã äàõü ò¯¯õ, ñΨëûí ¯ë õªäëªõ äóðñãàë yII äýâòýð ÃÎâÜѯÌÁÝÐ, äÀðÕÀÍ-ÓÓË, ÎðÕÎÍ Àéìàã 1 ÃÎÂÜѯÌÁÝÐ ÀÉÌÃÈÉÍ ÍÓÒÀà ÄÀÕÜ Ò¯¯Õ, ÑΨËÛÍ ¯Ë ÕªÄËªÕ ÄÓÐÑÃÀË DDC 306 Ý-66 Зохиогч: Г.Энхбат б.ДаваацЭрЭн Гэрэл зургийг: б.ДаваацЭрЭн П.Чинбат Гар зургийг: б.ДаваацЭрЭн Дизайнер: б.аЛТАНСҮх Орчуулагч: ц.цОЛМОн Зохиогчийн эрх хамгаалагдсан. © 2012, Copyrigth © 2012 by the Center of Cultural Соёлын өвийн төв, Улаанбаатар, Монгол улс Heritage, Ulaanbaatar, Mongolia Энэхүү цомгийг Соёлын өвийн төвийн зөвшөөрөлгүйгээр бүтнээр нь буюу хэсэгчлэн хувилан олшруулахыг хориглоно. Монгол улс Улаанбаатар хот - 211238 Сүхбаатар дүүрэг Сүхбаатарын талбай 3 Соёлын төв өргөө б хэсэг Соёлын өвийн төв Шуудангийн хайрцаг 223 веб сайт: www.monheritage.mn и-мэйл: [email protected] Утас: 976-11-312735 ISBN 978-99929-61-98-8 боловсрол, Соёл, Соёлын өвийн төв Говьсүмбэр аймгийн Дархан-Уул аймгийн Орхон аймгийн Шинжлэх ухааны яам ЗДтГазар ЗДтГазар ЗДтГазар 2 ÃÎÂÜѯÌÁÝÐ ÀÉÌÃÈÉÍ ÍÓÒÀà ÄÀÕÜ Ò¯¯Õ, ÑΨËÛÍ ¯Ë ÕªÄËªÕ ÄÓÐÑÃÀË ÃÀÐ×Èà Өмнөх үг 4 Удиртгал 5 Говьсүмбэр аймгийн нутаг дахь түүх, соёлын үл хөдлөх дурсгалын тухай 19 Говьсүмбэр аймгийн нутаг дахь түүх, соёлын үл хөдлөх дурсгалын байршил 23 Говьсүмбэр аймгийн нутаг дахь түүх, соёлын үл хөдлөх дурсгалууд -

SEMI ANNUAL REPORT (Oct 1, 2017 - Mar 31, 2018)
Mercy Corps LTS2 Semi-annual Report (October 1, 2017 to March 31, 2018) Leveraging Tradition and Science in Disaster Risk Reduction in Mongolia-2 (LTS2 - Mongolia) SEMI ANNUAL REPORT (Oct 1, 2017 - Mar 31, 2018) Agreement # AID-OFDA-G-15-00101 Submitted to: USAID Submitted by: Mercy Corps April 2018 COUNTRY CONTACT HEADQUARTERS CONTACT Ramesh Singh Denise Ledgerwood Country Director Senior Program Officer Mercy Corps Mercy Corps PO Box 761 45 SW Ankeny Street Ulaanbaatar 79, Mongolia Portland, OR 97204 Phone: +976 9911 4204 Phone: +1.503.896.5000 [email protected] [email protected] Mercy Corps LTS2 Semi-annual Report (Oct 1, 2017 – March 31, 2018) ACRONYMS AND TRANSLATIONS Aimag An administrative unit similar to a province or state APF Aimag Partnership Facilitator AWI Advanced Weather Information Service Bagh An administrative unit similar to a sub-county (sub-soum) CITA Communication Information and Technology Authority Dzud An environmental hazard that unfolds over several seasons and includes drought conditions in the summer leading to poor forage availability and low temperatures, heavy snows and/or ice in winter, which combine to exhaust animals, leading to death from starvation or exposure. ECHO European Civil Protection and Humanitarian Aid Operations ES engageSPARK EMA Emergency Management Agency FAO Food and Agriculture Organization of the United Nations Hural An elected decision-making body at the district, province and national level ICT Information and Communication Technology KEIO Keio University of Japan LEWS -

Carbon Finance in Mongolia
1 Capacity Building for Development and Implementation of Carbon Finance Projects (CBDICFP) WORLD BANK CARBON FINANCE IN MONGOLIA Mongolia 2011 2 This report “ Carbon Finance in Mongolia “ has been prepared and published within the project on capacity building for development and implementation of carbon finance projects in Mongolia financed by Policy and Human Resource Development Fund of Japan and imple- mented between April 2008- December 2010 jointly by Ministry of Nature, Environment and Tourism, Mongolia and the World Bank. Copyright © 2011, Ministry of Nature, Environment and Tourism, Mongolia Disclaimers The content and views expressed in this publication do not necessarily reflect the views or policies of the Government of Mongolia and the World Bank. Ministry of Nature, Environment and Tourism of Mongolia will not guarantee the accuracy or completeness of any information published herein, and thus will not be responsible for any losses or damages arising from the use of this information or from any errors or omissions therein. This publication may be reproduced in whole or in part in any form for educational or non- profit services without special permission from the copyright holder, provided acknowledge- ment of the source is made. 3 CONTENT Volume 1: The CDM in Mongolia INTRODUCTION .......................................... 10 PDDs under development ................................. 33 BACKGROUND ............................................. 10 Annex 4: PROJECT OPPORTUNITIES .......... 39 Concepts and PINs ......................................... -

RANGELAND GOVERNANCE in a SUBURBAN AREA of POST-SOCIALIST MONGOLIA Takahiro Tomita Kinugasa Research Organization, Ritsumeikan
“The authors agree to allow the Digital Library of the Commons to add this paper to its archives for IASC conferences.” RANGELAND GOVERNANCE IN A SUBURBAN AREA OF POST-SOCIALIST MONGOLIA Takahiro Tomita Kinugasa Research Organization, Ritsumeikan University Kyoto, Japan E-mail: [email protected] The way in which to ensure compatibility between development and environment preservation is one of the most crucial issues for pastoral societies. In suburban areas of the capital and secondary cities of Mongolia, the influx of herders who have migrated in search of a better life after Mongolia transitioned from a socialist to a market economy in the early 1990s, has caused problems in rangelands such as pasture degradation by overgrazing and shortages of water and forest resources. To address this problem, the “Peri-Urban Rangeland Project” was launched in 2008 by the Mongolian government with the help of funding from the U.S. The key objective of this project was to determine a sustainable environmental and economic model for the pastoral economy by changing the extensive, nomadic pastoral economy into an intensive, sedentary one. However, the specific objective of the project, which involves admitting a small number of herders to use land exclusively for long periods, differs from Mongolia’s tradition of open access pasture use that enables co-management of multiple, overlapping, and contingent resources. For example, in Orkhon district, a suburban area in Bulgan province, Mongolia, which is one of the project sites, many herder groups have already signed pasture land use contracts for fifteen years, and this has generated a new problem that these plots overlap with other herders’ seasonal campsites and public meadows. -

2016 EITI Report, MSG Agreed to Have MNT 100 Million As a Quasi-Fiscal Expenditure Materiality Threshold for Soes As an Experiment
MONGOLIA EXTRACTIVE INDUSTRIES TRANSPARENCY INITIATIVE MONGOLIA ELEVENTH EITI RECONCILIATION REPORT 2016 NOVEMBER 2017 © 2017 KPMG Audit LLC, a Mongolian member firm of the KPMG network of independent member firms affiliated with KPMG International Cooperative (“KPMG International”), a Swiss entity. All rights reserved. 1 Table of contents Glossary 6 1 Introduction 9 1.1 Introduction 9 1.2 Data assurance of the 2016 M.EITI Report 10 1.3 Participants in the 2016 M.EITI Report 10 1.4 Acknowledgement 10 2 Executive Summary 11 2.1 Overview of approach and reconciliation results 11 2.2 Summary of government receipts reconciled 12 2.3 Key findings 16 3 Reconciliation Scope and Methodology 18 3.1 Introduction 18 3.2 Reconciliation methodology 20 3.3 Reconciliation approach 25 3.4 Reporting entities and receipts to be covered 34 4 Reconciliation Results 36 4.1 Reconciliation results 36 4.2 Unresolved differences and unreported companies (REQ 4.9) 42 4.3 Accounting framework and external audit 46 5 Extractive Industries in Mongolia 47 5.1 Overview of the Extractive Industry 47 5.2 EI licences 81 5.3 Distribution of revenue 98 5.4 State participation in EI 109 5.5 Other matters 127 6 Recommendations 147 6.1 Implementation of last year’s recommended actions (REQ 7.3) 147 6.2 IA’s Recommendations for future M.EITI Reports 148 6.3 MSG’s Recommendations 150 7 Appendices 152 © 2017 KPMG Audit LLC, a Mongolian member firm of the KPMG network of independent member firms affiliated with KPMG International Cooperative (“KPMG International”), a Swiss entity. -

Assessment of Wood Product Value Chains and Recommendations For
Assessment of Wood Product Value Chains and Recommendations for the Mongolian Wood-Processing Industry Firewood in front of a school in Erdenebulgan soum, Khuvsgul aimag, Photo: R.Glauner Consultancy Report Reinhold Glauner Delgerjargal Dugarjav Final Table of Content Executive Summary ................................................................................................................................ vii 1 Introduction and Objectives ............................................................................................................ 1 2 Methods .......................................................................................................................................... 2 2.1 Study Limitations ................................................................................................................... 2 2.2 Value-Chain Analysis .............................................................................................................. 2 2.3 Supply and Demand Analyses ................................................................................................ 4 2.4 Market Analysis ...................................................................................................................... 4 2.5 Market Constraints and Industry Recommendations ............................................................ 5 2.5.1 Private Sector Partners ...................................................................................................... 5 2.5.2 Focus Groups, Consultation, and Review Workshops