US5523508.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alfa Olefins Cas N
OECD SIDS ALFA OLEFINS FOREWORD INTRODUCTION ALFA OLEFINS CAS N°:592-41-6, 111-66-0, 872-05-9, 112-41-4, 1120-36-1 UNEP PUBLICATIONS 1 OECD SIDS ALFA OLEFINS SIDS Initial Assessment Report For 11th SIAM (Orlando, Florida, United States 1/01) Chemical Name: 1-hexene Chemical Name: 1-octene CAS No.: 592-41-6 CAS No.: 111-66-0 Chemical Name: 1-decene Chemical Name: 1-dodecene CAS No.: 872-05-9 CAS No.: 112-41-4 Chemical Name: 1-tetradecene CAS No.: 1120-36-1 Sponsor Country: United States National SIDS Contract Point in Sponsor Country: United States: Dr. Oscar Hernandez Environmental Protection Agency OPPT/RAD (7403) 401 M Street, S.W. Washington, DC 20460 Sponsor Country: Finland (for 1-decene) National SIDS Contact Point in Sponsor Country: Ms. Jaana Heiskanen Finnish Environment Agency Chemicals Division P.O. Box 140 00251 Helsinki HISTORY: SIDS Dossier and Testing Plan were reviewed at the SIDS Review Meeting or in SIDS Review Process on October 1993. The following SIDS Testing Plan was agreed: No testing ( ) Testing (x) Combined reproductive/developmental on 1-hexene, combined repeat dose/reproductive/developmental on 1-tetradecene and acute fish, daphnid and algae on 1- tetradecene. COMMENTS: The following comments were made at SIAM 6 and have been incorporated in this version of the SIAR: 2 UNEP PUBLICATIONS OECD SIDS ALFA OLEFINS 1. The use of QSAR calculations for aquatic toxicity, 2. More quantitative assessment of effects; and 3. Provide more details for each endpoint. The following comments were made at SIAM 6, but were not incorporated into the SIAR for the reasons provided: 1. -

Wide Range Linear Alpha Olefin Processes
IHS Chemical Process Economics Program Consolidated Report CR001 Wide Range Linear Alpha Olefin Processes By Marianna Asaro July 2014 ihs.com/chemical IHS Chemical Process Economics Program Consolidated Report | CR001 IHS Chemical agrees to assign professionally qualified personnel to the preparation of the Process Economics Program’s reports and will perform the work in conformance with generally accepted professional standards. No other warranties expressed or implied are made. Because the reports are of an advisory nature, neither IHS Chemical nor its employees will assume any liability for the special or consequential damages arising from the Client’s use of the results contained in the reports. The Client agrees to indemnify, defend, and hold IHS Chemical, its officers, and employees harmless from any liability to any third party resulting directly or indirectly from the Client’s use of the reports or other deliverables produced by IHS Chemical pursuant to this agreement. For detailed marketing data and information, the reader is referred to one of the IHS Chemical programs specializing in marketing research. The IHS CHEMICAL ECONOMICS HANDBOOK Program covers most major chemicals and chemical products produced throughout the world. In addition the IHS DIRECTORY OF CHEMICAL PRODUCERS services provide detailed lists of chemical producers by company, product, and plant for the United States, Europe, East Asia, China, India, South & Central America, the Middle East & Africa, Canada, and Mexico. July 2014 ii © 2014 IHS IHS Chemical Process Economics Program Consolidated Report | CR001 PEP Report CR001 Wide Range Linear Alpha Olefin Processes By Marianna Asaro July 2014 Abstract This report consolidates and updates the Process Economics Program’s technical and economic analyses of wide-range linear alpha olefins (LAO) manufacturing technologies since PEP first reported on the subject in the 1960s. -

United States Patent (19) 11 3,968,133 Knifton (45) July 6, 1976
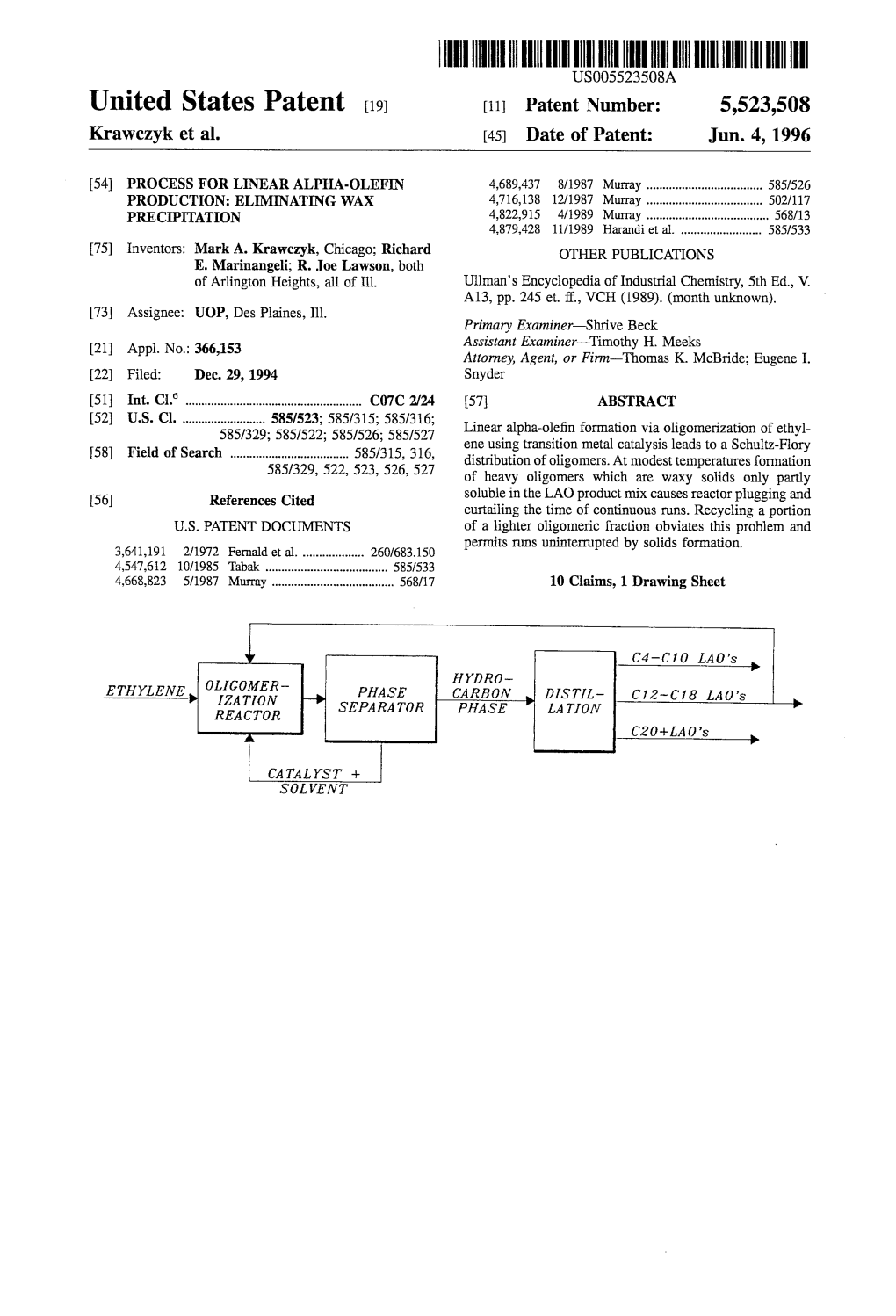
United States Patent (19) 11 3,968,133 Knifton (45) July 6, 1976 54 CARBOXYLATION PROCESS FOR 3,723,486 3/1973 Kajimoto...................... 260/410.9 R PREPARING LINEAR FATTY ACDS OR 3,776,929 12/1973 Mrowca....................... 260/410.9 R ESTERS 3,819,669 6/1974 Knifton........................ 260/410.9 R 3,832,391 8/1974 Parshall........................ 260/410.9 R (75) Inventor: John F. Knifton, Poughouag, N.Y. Primary Examiner-Patrick P. Garvin 73 Assignee: Texaco Inc., New York, N.Y. Assistant Examiner-John F. Niebling 22) Filed: Nov. 25, 1974 Attorney, Agent, or Firm-T. H. Whaley; C. G. Ries; (21) Appl. No.: 526,867 Bernard Marlowe 52) U.S. C. ......................... 260/410.9 R; 260/413; 57 ABSTRACT 260/497 A; 260/488 K; 260/491; 260/.532; This invention concerns a process for preparing linear 260/533 A fatty (carboxylic) acids and esters from the reaction of 51 Int. Cl’............................................ C11C 3/02 olefins, alcohols (or water) and carbon monoxide. The (58) Field of Search......... 260/410.9 R, 413, 497 A, process is catalyzed by catalytic amounts of disper 260/488 K, 491, 532,533 A sions of ligand-stabilized, palladium(II) halide com plexes, in quaternary ammonium, phosphonium or ar 56 References Cited sonium salts of trihalostannate(II) or trihaloger UNITED STATES PATENTS manate(II). These empirically selected palladium cata 3,437,676 4/1969 Kutepow...................... 260/410.9 R lysts can be recycled with fresh olefin feed several 3,530, 155 9/1970 Fenton......................... 260/410.9 R times before substantial loss of catalytic activity is 3,530,168 9/1970 Biale..... -

Process Economics Program (PEP): Wide Range Linear Alpha Olefin Processes & On-Purpose Linear Alpha Olefin Processes PEP CR001 & CR002 Prospectus
IHS CHEMICAL Process Economics Program (PEP): Wide Range Linear Alpha Olefin Processes & On-Purpose Linear Alpha Olefin Processes PEP CR001 & CR002 Prospectus IHS CHEMICAL PROSPECTUS PEP CR001 & CR002 Contents Contents ..................................................................................................................................... 2 Introduction ................................................................................................................................ 3 Abstract ...................................................................................................................................... 5 Key Questions Addressed in the Reports .................................................................................. 6 Deliverables ............................................................................................................................... 6 Process Technologies Reviewed in CR001 & CR002 ................................................................ 6 Table of Contents ....................................................................................................................... 7 CR001 - Wide Range Linear Alpha Olefin Processes ................................................................ 7 CR002 - On-Purpose Linear Alpha Olefin Processes .............................................................. 10 Meet the Author........................................................................................................................ 15 About the IHS Chemical Process -

(12) United States Patent (10) Patent No.: US 6,787,576 B2 Kiss Et Al
USOO6787576B2 (12) United States Patent (10) Patent No.: US 6,787,576 B2 Kiss et al. (45) Date of Patent: Sep. 7, 2004 (54) LINEAR ALPHAOLEFINS FROM NATURAL 5,210,060 A 5/1993 Radlowski et al. ......... 502/202 GAS-DERVED SYNTHESIS GAS OVERA 5.248,701 A 9/1993 Soled et al. ................ 518/700 NONSHIFTING COBALT CATALYST 6,166.262 A 12/2000 Connor ....................... 568/460 (75) Inventors: Gabor Kiss, Hampton, NJ (US); Rocco FOREIGN PATENT DOCUMENTS Anthony Fiato, Basking Ridge, NJ GB 21696.14 A 7/1986 ............. CO7C/1/04 (US); Frank Hershkowitz, Liberty JP 61O18433 A 1/1986 ............ B01J/21/16 Corner, NJ (US); David Chester Long, NL 7712952 A 5/1979 ............ BO1J/23/74 Ashburn, VA (US) OTHER PUBLICATIONS (73) Assignee: ExxonMobil Research and R. Madon et al., “Primary and Secondary Reaction Path Engineering Company, Annandale, NJ ways in Ruthenium-Catalyzed Hydrocarbon Synthesis”, (US) The Journal of Physical Chemistry, 1991, 95, pp. 7795-7804. (*) Notice: Subject to any disclaimer, the term of this E. Iglesia et al., “Transport-Enhanced C-Olefin Readsorp patent is extended or adjusted under 35 tion Pathways in Ru-Catalyzed Hydrocarbon Synthesis', U.S.C. 154(b) by 0 days. Journal of Catalysis, vol. 129, pp. 238-256 (1991). R. J. Madon et al., “Non-Flory Product Distributions in (21) Appl. No.: 10/330,860 Fischer-Tropsch Synthesis Catalyzed by Ruthenium, Cobalt, and Iron”, ACS Symposium Series Selectivity in (22) Filed: Dec. 27, 2002 Catalysis, Editors: M. E. Davis and S. L. Suib, New York, (65) Prior Publication Data Aug. 25–30, 1991. -

Linear Alpha-Olefins (681.5030)
IHS Chemical Chemical Economics Handbook Linear alpha-Olefins (681.5030) by Elvira O. Camara Greiner with Yoshio Inoguchi Sample Report from 2010 November 2010 ihs.com/chemical November 2010 LINEAR ALPHA-OLEFINS Olefins 681.5030 B Page 2 The information provided in this publication has been obtained from a variety of sources which SRI Consulting believes to be reliable. SRI Consulting makes no warranties as to the accuracy completeness or correctness of the information in this publication. Consequently SRI Consulting will not be liable for any technical inaccuracies typographical errors or omissions contained in this publication. This publication is provided without warranties of any kind either express or implied including but not limited to implied warranties of merchantability fitness for a particular purpose or non-infringement. IN NO EVENT WILL SRI CONSULTING BE LIABLE FOR ANY INCIDENTAL CONSEQUENTIAL OR INDIRECT DAMAGES (INCLUDING BUT NOT LIMITED TO DAMAGES FOR LOSS OF PROFITS BUSINESS INTERRUPTION OR THE LIKE) ARISING OUT OF THE USE OF THIS PUBLICATION EVEN IF IT WAS NOTIFIED ABOUT THE POSSIBILITY OF SUCH DAMAGES. BECAUSE SOME STATES DO NOT ALLOW THE EXCLUSION OR LIMITATION OF LIABILITY FOR CONSEQUENTIAL OR INCIDENTAL DAMAGES THE ABOVE LIMITATION MAY NOT APPLY TO YOU. IN SUCH STATES SRI CONSULTING’S LIABILITY IS LIMITED TO THE MAXIMUM EXTENT PERMITTED BY SUCH LAW. Certain statements in this publication are projections or other forward-looking statements. Any such statements contained herein are based upon SRI Consulting’s current knowledge and assumptions about future events including without limitation anticipated levels of global demand and supply expected costs trade patterns and general economic political and marketing conditions. -

Metallocene Polyalpha Olefins (Mpaos) Process Economics Program Report 296
` IHS CHEMICAL Metallocene Polyalpha Olefins (mPAOs) Process Economics Program Report 296 December 2016 ihs.com PEP Report 296 Metallocene Polyalpha Olefins (mPAOs) Gajendra Kumar Principal Analyst Tony Pavone Sr. Principal Analyst Downloaded 20 December 2016 10:10 AM UTC by Ellen Blue, IHS INC ([email protected]) IHS Chemical | PEP Report 296 Metallocene Polyalpha Olefins (mPAOs) PEP Report 296 Metallocene Polyalpha Olefins (mPAOs) Gajendra Kumar, Principal Analyst Tony Pavone, Sr. Principal Analyst Abstract Polyalpha olefins (PAOs) represent a family of primarily decene-1 oligomers (mostly trimers) that have found wide use as a fully synthetic lubricant base oil component. PAOs provide superior lubricant properties in viscosity, viscosity index, cold cranking capability, emulsion resistance, lower pour point, lubricity, friction reduction, low volatility in use, higher flash temperature, and thermal and oxidative stability. The downside is lower solvency and biodegradation, poorer seal swell, and higher cost. The American Petroleum Institute (API) designates PAO components as Group-4 fully synthetic basestock. Periodic shortages in decene-1 LAO (linear alpha olefin) feedstock availability have forced PAO producers to occasionally blend C10 with both lighter (C8) and heavier (C12) LAO feedstocks to produce PAO with adequate physical and performance properties. The oligomerization process is not very selective, and produces a reactor product containing LAO dimers, trimers, tetramers, and pentamers, plus unreacted feedstock. While originally developed to produce fully synthetic basestock for conventional motor oil requiring 4 centistoke (cSt) viscosity, additional product grades with different viscosities have been supplemented with newer PAO components of much higher viscosity (100+ cSt) that have found widespread use as blend stock components in heavy duty gear boxes, transfer cases, and transmissions, such as those found in wind turbine generators. -

(12) United States Patent (10) Patent No.: US 8,961,775 B2 Joshi Et Al
US008961775B2 (12) United States Patent (10) Patent No.: US 8,961,775 B2 Joshi et al. (45) Date of Patent: Feb. 24, 2015 (54) HIGH PRODUCTIVITY KOLBE REACTION (2013.01): CI0G 3/42 (2013.01): CI0G 3/44 PROCESS FORTRANSFORMATION OF (2013.01): CI0G 3/48 (2013.01); C25B 3/10 FATTY ACDS DERVED FROM PLANT OIL (2013.01): CI0G 45/58 (2013.01): CI0G 45/64 AND ANIMAL EAT (2013.01); Y02T 50/678 (2013.01); Y02E 50/13 (2013.01); C07C 2521/04 (2013.01); C07C (71) Applicant: Altranex Corporation, Scarborough 2521/08 (2013.01); C07C2523/28 (2013.01); (CA) (Continued) (72) Inventors: Chandrashekhar H. Joshi, Kingston (58) Field of Classification Search (CA); Graham Thomas Thornton USPC ............................... 585/1: 205/413, 415, 462 Gibson, Kingston (CA); Dzmitry See application file for complete search history. Malevich, Kingston (CA); Michael (56) References Cited Glenn Horner, West Roxbury, MA (US) U.S. PATENT DOCUMENTS (73) Assignee: Altranex Corporation (CA) 4,018,844 A 4, 1977 MereSZ et al. (*) Notice: Subject to any disclaimer, the term of this 7,198,710 B2 4/2007 Miller et al. patent is extended or adjusted under 35 (Continued) U.S.C. 154(b) by 0 days. OTHER PUBLICATIONS (21) Appl. No.: 14/331,390 B.C.L. Weedon, "Anodic Syntheses With Carboxylic Acids,” Quar (22) Filed: Jul. 15, 2014 terly Reviews of Chemical Society, 1952, vol. 6 pp. 380-398. (Continued) (65) Prior Publication Data US 2014/O323784 A1 Oct. 30, 2014 Primary Examiner — Ellen McAvoy (74) Attorney, Agent, or Firm — American Patent Agency Related U.S. -

Selective Production of Bio-Based Linear Alpha-Olefin from Wasted
polymers Article Selective Production of Bio-Based Linear Alpha-Olefin from Wasted Fatty Alcohol on Al2O3 for Bio-Based Chemicals Hye-Jin Lee 1,2, Il-Ho Choi 1 , Seung-Wook Kim 2 and Kyung-Ran Hwang 1,* 1 Energy Resource Upcycling Research Laboratory, Korea Institute of Energy Research, Daejeon 34129, Korea; [email protected] (H.-J.L.); [email protected] (I.-H.C.) 2 Department of Chemical and Biological Engineering, Korea University, Seoul 136701, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-42-860-3504 Abstract: The catalytic dehydration of a bio-based fatty alcohol was performed using Al2O3 prepared by solvothermal synthesis for selective production of long-chain linear-alpha-olefins (LAO). The effect of the synthesis temperature of alumina precursors on the dehydration of 1-octadecanol (C18H38O) was examined based on the textural properties and Lewis acid–base properties of the catalysts. Amorphous alumina synthesized at 325 ◦C showed the highest surface area (233.07 m2/g) and total pore volume (1.237 cm3/g) among the catalysts and the best dehydration results: 93% conversion, 62% selectivity of 1-octadecene (C18H36), and 89% LAO purity. This was attributed to the increased Al/O ratio and atomic concentration of surface O in alumina, which were important factors in the catalytic dehydration of 1-octadecanol through the synergistic catalysis of acid–base pairs. The produced bio-based LAO can be key intermediates for synthesis of oxo alcohols and poly-alpha-olefins, as alternatives to petroleum-based LAO to achieve carbon neutrality in chemical industry. -

Light Linear Alpha Olefin Market Study
IHS CHEMICAL Light Linear Alpha Olefin Market Study Special Report Prospectus IHS CHEMICAL Contents Contents ..................................................................................................................................... 2 Introduction ................................................................................................................................ 3 Study Scope ............................................................................................................................... 5 Key Questions Addressed in the Study ...................................................................................... 7 Deliverables ............................................................................................................................... 8 Proposed Table of Contents ...................................................................................................... 9 Methodology ............................................................................................................................ 11 Study Team .............................................................................................................................. 15 Qualifications............................................................................................................................ 18 About IHS Chemical ................................................................................................................. 21 About IHS ................................................................................................................................ -

Light Linear Alpha Olefin Market Study
Light Linear Alpha Olefin Market Study Chemical Strategic Report Prospectus Light Linear Alpha Olefin Market Study Light Linear Alpha Ole n Market Study Light Linear Alpha Ole n Market Study Contents Introduction The linear alpha olefin (“LAO”) business is complex, serving a broad range of industries and Introduction ............................................................................................. 3 applications therein from commodity plastics to small volume fine and performance chemicals. Study Scope ............................................................................................. 5 A simplified map of the LAO value chain is given below: Key Questions Addressed in the Study ............................................... 7 Deliverables .............................................................................................. 8 Alpha Olefins Supply Chain Proposed Table of Contents ................................................................. 9 Full Range Ethylene Polybutene-1 Processes Butene-1 Methodology .......................................................................................... 11 PE/PP Hexene-1 Study Team ............................................................................................ 15 Comnomer Dimerization Plasticizer Alcohols Qualifications ......................................................................................... 18 Octene-1 Polyolefin Elastomers Trimerization About Chemical at IHS Markit ............................................................. 21 -

WO 2007/050746 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date (10) International Publication Number 3 May 2007 (03.05.2007) PCT WO 2007/050746 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every ClOL 1/18 (2006.01) ClOL 10/16 (2006.01) kind of national protection available): AE, AG, AL, AM, ClOL 1/185 (2006.01) AT,AU, AZ, BA, BB, BG, BR, BW, BY, BZ, CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DZ, EC, EE, EG, ES, FI, (21) International Application Number: GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, PCT/US2006/041768 JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LV,LY,MA, MD, MG, MK, MN, MW, MX, MY, (22) International Filing Date: 26 October 2006 (26.10.2006) MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, 11/260,524 27 October 2005 (27.10.2005) US ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AT,BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, (71) Applicant (for all designated States except US): FR, GB, GR, HU, IE, IS, IT, LT, LU, LV,MC, NL, PL, PT, CHEVRON PHILLIPS CHEMICAL COMPANY RO, SE, SI, SK, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, LP [US/US]; 10001 Six Pines Drive, The Woodlands, TX GN, GQ, GW, ML, MR, NE, SN, TD, TG).