CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
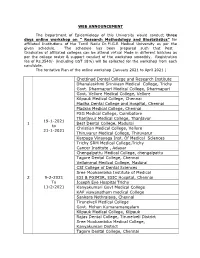
Research Methodology and Biostatistics” for Affiliated Institutions of the Tamil Nadu Dr.M.G.R Medical University As Per the Given Schedule
WEB ANNOUNCEMENT The Department of Epidemiology of this University would conduct three days online workshop on “ Research Methodology and Biostatistics” for affiliated Institutions of the Tamil Nadu Dr.M.G.R Medical University as per the given schedule. The schedule has been prepared such that Post Graduates of affiliated colleges can be attend virtual Mode in different batches as per the college roster & support conduct of the workshop smoothly. Registration fee of Rs.3540/- (including GST 18%) will be collected for the workshop from each candidate. The tentative Plan of the online workshop (January 2021 to April 2021 ) Chettinad Dental College and Research Institute Dhanalaskhmi Srinivasn Medical College, Trichy Govt. Dharmapuri Medical College, Dharmapuri Govt. Vellore Medical College, Vellore Kilpauk Medical College, Chennai Madha Dental College and Hospital, Chennai Madras Medical College, Chennai PSG Medical College, Coimbatore Thanjavur Medical College, Thanjavur 19-1-2021 1 Best Dental College, Madurai to Christian Medical College, Vellore 21-1-2021 Thiruvarur Medical College, Thiruvarur Karpaga Vinayaga Inst. Of Medical Sciences Trichy SRM Medical College,Trichy Cancer Institute , Adayar Chengalpattu Medical College, chengalpattu Tagore Dental College, Chennai Vellammal Medical College, Madurai CSI College of Dental Sciences Sree Mookambika Institute of Medical 29-2-2021 ESI & PGIMSR, ESIC Hospital, Chennai To Joseph Eye Hospital Trichy 11-2-2021 Kanyakumari Govt Medical College KAP viswanatham medical College Sankara Nethralaya, Chennai Tirunelveli Medical College Govt. Mohan Kumaramangalam Kilpauk Medical College, Kilpauk Rajas Dental College, Tirunelveli District Sree Mookambika Medical College, Kanyakumari District Tagore Dental College, Chennai Trichy SRM Medical College,Trichy Vivekanandha Dental College for Women, Namakkal Christian medical College, Vellore Govt. -

ALLOTTED LIST (1).Xlsx
PG DEGREE/DIPLOMA COURSES 2020-2021 SESSION RESULT- ROUND II - GOVERNMENT QUOTA SNO GRANK ARNO NAME COMMUNITY SERVICE MARKSFROM TO M.D. D.V.L M.D. D.V.L 1 1 5709 BHUVANANDHINI K BC Service 1035.05 MADRAS MEDICAL COLLEGE, CHENNAI MADRAS MEDICAL COLLEGE, CHENNAI M.D. Radio Diagnosis M.D. Radio Diagnosis 2 2 5272 SHIVA A MBC/DNC Service 1002.3 MADRAS MEDICAL COLLEGE, CHENNAI MADRAS MEDICAL COLLEGE, CHENNAI M.D. Radio Diagnosis M.D. Radio Diagnosis 3 3 4624 B KIRUBAKARAN BC Service 991.2 MADRAS MEDICAL COLLEGE, CHENNAI MADRAS MEDICAL COLLEGE, CHENNAI M.D. Radio Diagnosis M.D. Radio Diagnosis 4 5 3713 SIDDHARTH P BC Service 940 COIMBATORE MEDICAL COLLEGE, COIMBATORE COIMBATORE MEDICAL COLLEGE, COIMBATORE M.D. Radio Diagnosis M.D. Radio Diagnosis 5 6 3634 LENINPRABHU P MBC/DNC Service 934.72 STANLEY MEDICAL COLLEGE , CHENNAI STANLEY MEDICAL COLLEGE , CHENNAI M.D. D.V.L M.D. D.V.L 6 7 4294 ELANGO M MBC/DNC Service 931.5 STANLEY MEDICAL COLLEGE , CHENNAI STANLEY MEDICAL COLLEGE , CHENNAI M.D. Radio Diagnosis M.D. Radio Diagnosis 7 9 6403 RAGAVI MBC/DNC Service 930.91 STANLEY MEDICAL COLLEGE , CHENNAI STANLEY MEDICAL COLLEGE , CHENNAI M.D. Radio Diagnosis M.D. Radio Diagnosis 8 10 5180 SHYAMALA M BC Service 930.35 STANLEY MEDICAL COLLEGE , CHENNAI STANLEY MEDICAL COLLEGE , CHENNAI ANTONY SHERLY M.D. General Medicine M.D. General Medicine 9 16 5772 BC Service 918.21 ROSE M MADURAI MEDICAL COLLEGE, MADURAI MADURAI MEDICAL COLLEGE, MADURAI M.D. General Medicine M.D. General Medicine 10 17 5323 C ADITHYAN BC Service 914.4 MADRAS MEDICAL COLLEGE, CHENNAI MADRAS MEDICAL COLLEGE, CHENNAI M.D. -

E960 Public Disclosure Authorized
Health care Waste Management System E960 Public Disclosure Authorized TNHSDP Public Disclosure Authorized Health Care Waste Management System Public Disclosure Authorized Public Disclosure Authorized Health care Waste Management System Health Care Waste Management System The Government of Tamil Nadu has proposed to implement a Health Systems Development Project (HSDP) with the World Bank assistance. The main objective of the HSDP is to improve the health outcomes of the people of Tamil Nadu with special reference to poor especially in remote and inaccessible areas. The project objectives are in keeping with the Millennium Development Goals. The HSDP envisages a substantial improvement in health infrastructure at the secondary level institutions. Several interventions have been planned for reducing infant, child and maternal mortality and morbidity. The project would also develop effective models to combat non communicable diseases like diabetes, hypertension, cardio vascular diseases, cancer etc. HSDP also plans to develop a comprehensive Health Management Information System in addition to capacity building of the health functionaries. The project would attempt to initiate bring a fruitful Public Private Partnership in health sector. The Govt. of Tamil Nadu has analysed the present scenario of bio medical waste management right from tertiary institutions to the PHCs. The Bio Medical waste management in private sector has also been analysed. The bio medical waste generated by the health institutions both in public and private sector needs to be regulated under the Bio-Medical Waste (Management and Handling) (Second Amendment) Rules 2000, Ministry of Environment and Forest, Govt. of India Notification New Delhi. Under the health care waste management plan Govt. -

Directorate of Medical Education
1 Directorate of Medical Education Hand Book on Right to Information Act - 2005 2 CHAPT ER DETAILS PAGE NO NO. 1. INTRODUCTION 3 2 PARTICULARS OF ORGANISATION, FUNCTIONS AND DUTIES 5 3 POWERS AND DUTIES OF OFFICERS AND EMPLOYEES 80 4 RULES, REGULATIONS,INSTRUCTIONS, MANUAL AND RECORDS 91 FOR DISCHARGING FUNCTIONS 5 PARTICULARS OF ANY ARRANGEMENT THAT EXISTS FOR 93 CONSULTATION WITH OR REPRESENTATION BY THE MEMBERS OF THE PUBLIC IN RELATION TO THE FORMULATION OF ITS POLICY OR IMPLEMENTATION THEREOF 6 A STATEMENT OF THE CATEGORIES OF DOCUMENTS THAT ARE 94 HELD BY IT OR UNDER ITS CONTROL 7 A STATEMENT OF BOARDS, COUNCIL, COMMITTEES AND OTHER 95 BODIES CONSTITUTED AS ITS PART 8 THE NAMES, DESIGNATION AND OTHER PARTICULARS OF THE 96 PUBLIC INFORMATION OFFICER 9 PROCEDURE FOLLOWED IN DECISION MAKING PROCESS 107 10 DIRECTORY OF OFFICERS AND EMPLOYEES 108 11 THE MONTHLY REMUNERATION RECEIVED BY EACH OF ITS 110 OFFICERS AND EMPLOYEES INCLUDING THE SYSTEM OF COMPENSATION AS PROVIDED IN REGULATIONS. 12 THE BUDGET ALLOCATED TO EACH AGENCY/OFFICERS 111 (PARTICULARS OF ALL PLANS, PROPOSED EXPENDITURES AND REPORTS ON DISBURSEMENT MADE 116 13 THE MANNER OF EXECUTION OR SUBSIDY PROGRAMME 14 PARTICULARS OF RECEIPIENTS OF CONCESSIONS, PERMITS OR 117 AUTHORISATION GRANTED BY IT 15 NORMS SET BY IT FOR THE DISCHARGE OF ITS FUNCTIONS 118 16 INFORMATION AVAILABLE IN AN ELECTRONIC FORM 119 17 PARTICULARS OF THE FACILITIES AVAILABLE TO CITIZENS FOR 120 OBTAINING THE INFORMATION 18 OTHER USEFUL INFORMATION 121 3 CHAPTER-1 - INTRODUCTION 1.1This handbook is brought out by the Directorate of Medical Education (Government of Tamil Nadu) , Chennai as required by the Right to Information Act, 2005. -
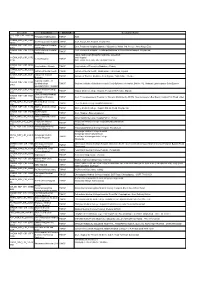
Circuit ID Location District Customer Name SDC NSP CIR 240B1AB Perungudi HUB Location TNHSP HUB D CHENN NSP CIR 33EF Govt.Royepettah Hospital TNHSP Govt
Circuit ID Location District Customer Name SDC_NSP_CIR_240B1AB Perungudi HUB location TNHSP HUB D CHENN_NSP_CIR_33EF Govt.Royepettah Hospital TNHSP Govt. Royepettah Hospital Royapettah DFDF CHENN_NSP_CIR_127F Govt.Peripheral Hospital TNHSP Govt. Peripheral Hospital,Opposite Vijayashree Mahal 3rd Avenue Anna Nagar East 4959 AnnaNagar CHENN_NSP_CIR_33195 Govt.Peripheral Hospital Govt. Peripheral Hospital - TondiarpetKailasam St New Washermanpet Royapuram TNHSP 01 Tondiarpet TAMIL NADU GOVERNMENT DENTAL COLLEGE CHENN_NSP_CIR_337D and Hospital Dental Hospital TNHSP 019B OPP. PARK STATION MUTHUSAMY SALAI CHENN_NSP_CIR_1B1D King Institute - Guindy TNHSP King Institute of Preventive Medicine - Guindy 3A6C CHENN_NSP_CIR_1ADA Institute of Mental health TNHSP Institute of Mental health ,Medavakkam Tank Road, Kilpauk C559 CHENN_NSP_CIR_102F Institute of Thoracic TNHSP Institute of Thoracic Medicine, Near Egmore Taluk Office, Chetpet 6663 Medicine Regional Institute of CHENN_NSP_CIR_283A Ophthalmology _ TNHSP Regional Institute of Ophthalmology & Govt. Ophthalmic Hospital ,Old No.132, Rukmani Lakshmipathy Salai Egmore 61F2 Govt.Ophthalmic Hospital CHENN_NSP_CIR_17427 Kilpauk Medical College- TNHSP Kilpauk Medical College Hospital, Periyavar EVR Salai, Kilpauk F0D Chennai Govt.Thiruvatteeswarar CHENN_NSP_CIR_3ABF Hospital for Thoracic TNHSP Govt. Thiruvatteeswarar Hospital for Thoracic Medicine,No 60/372 Near Aynavaram Bus Depot Konnur High Road Otteri 2EDA Medicine THENI_NSP_CIR_3540E Theni Medical College TNHSP Theni Medical College Hospital,kanavillaku -

KMC Chennai Alumni Association -Annual Report 2017-18
Annual Report 2017-18 KMC Chennai Alumni Association Govt Kilpauk Medical college Poonamalle High road,Kilpauk,Chennai India 600010 KMC Chennai Alumni Association -Annual report 2017-18 KMC Chennai Alumni Association Govt Kilpauk Medical College, Ponnamalle High Road, Kilpauk, Chennai-600 010 Reg No 70/2016 ( Central Chennai ) u/s 10 of the Tamilnadu Societies Registration Act 1975 Write to us at [email protected] Web site: www.kilpaukmedicalcollege.com KMC Chennai Alumni Association -Annual report 2017-18 Objects of the Association 1] To foster a continuing educational and philanthropic rela- tionship between Kilpauk Medical College alumni and the Col- lege, Hospital, its students, and faculty 2] To facilitate the meeting of Kilpauk Medical College alumni with one another 3] To encourage Kilpauk Medical College Alumni to make contributions to Society 4] To interact with foreign institutions and arrange exchange programs 5] To publish and help in publication and / or distributing peri- odicals, monthly magazines ,newspapers ,pamphlets, books for spread and advancements of recent medical improvements and in- novations 6] Scholarship aid to undergraduate and graduate students 7] Sponsorship of community service projects 8] Sponsorship of fund raising events which will benefit the College and Hospital directly or which will benefit the Alumni in the establishment of scholarships or to provide for the expenses of operating the Alumni KMC Chennai Alumni Association -Annual report 2017-18 Kilpauk Medical College Infrastructure development -

District Census Handbook, Madras, Part XIII a and B, Series-20
CENSUS OF INDIA 1981 SERIES 20 TAMIL NADU PART xm A and B DISTRICT CENSUS HANDBOOK TOWN DIRECTORY AND DIVISIONWISE PRIMARY CENSUS ABSTRACT MADRAS A. P. MUTHUSWAMI of the Indian Administrative Service Director of Census Operations Tamil Nadu 12-2,,_ A MADRAS DISTRICT KILOMETERS 012 ._.__.; ,. I NOTE: THE BOUNDARY OF MADRAS DISTRICT IS COTERMINOUS WITH THAT OF THE 26261 MADRAS CORPORATION --- ----- 27.. 32 40 616 \ I \ / ,.-j • I 34 , _/~--'i 26625 '. _- - - I \ ,~- " . " .1 33 \, J 35637 : \.. ...., i 75 } 28245 / " ~ I ( ~ 7 .lN~~~ I . " () 21_709 I 77 "'-- ~ ____ _ I I 38901 I 79 /. Bay Division Division I I 19698,' No. popLllation I '--80 -, ) ,---- J." ______ -r _~2lB__6~I~ 1O 20548 of " 6 ' \ I 109 , . 13 19617 I 39 ~40 I (, 110 5) 25661 __ ;' t e 21278 I.... __ -- I Bengal 15 14892 f'" \ I _- - 116 J \ , _-I 23 16 18319 , I 112 Ir- - -'II ,_ 9{'5 , 17 18090 /"33777>21537)' t-f-,r'lj -' J ' .... _ ( ,_ ..... -- I 115 :,7 169' 18 20930 '\ ..... _- \ 20 20114 / 47 18991 , 1--/ 48 19211 \ \ 113 , I 30422 49 22453 \ - - 411--/ 52 17427 ~~~ 56 18917 59 25388 61 16931 137 64 13871 -_ 25907 I 65 14935 _-_ I I 66 17385 (--- ,I 67 18620 147 I I 68 23619 22 771 _L..,, ____ -- 69 14531 , I 82 20480 r- ..... _""', J-_J • I 1 150 90 14118 \' 26230 & 1 ' ...... __ I __ /1 92 18780 .~ ~ \ ,.'. 94 19421 \ , 148 \ ,/ ::: 95 21592 • 24998 1/ .-._,.~. 96 13072 \. ./' 99 18665 \. / 102 2'2148 /" 129 19906 '. -
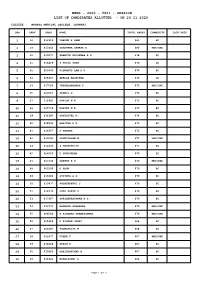
Mbbs - 2020 - 2021 - Session List of Candidates Allotted - on 23.11.2020
MBBS - 2020 - 2021 - SESSION LIST OF CANDIDATES ALLOTTED - ON 23.11.2020 COLLEGE : MADRAS MEDICAL COLLEGE, CHENNAI. SNO RANK ARNO NAME TOTAL MARKS COMMUNITY JOIN DATE 1 16 612916 VARUNN K SAMY 681 BC 2 19 617442 SARAVANA SANKAR B 680 MBC/DNC 3 26 610077 PRANITH KRISHNAA A G 678 BC 4 31 616829 S SELVA SREE 676 OC 5 32 620045 VISWANTH RAM K U 676 BC 6 33 615867 ADELLA AASRITHA 676 OC 7 34 617504 VENGADANADHAN P 675 MBC/DNC 8 35 603567 PRANIL G 675 BC 9 37 615981 KANIGA N N 675 BC 10 38 615558 ROHITH N D 675 BC 11 39 616397 SAKTHIVEL M 674 BC 12 40 618593 HARITHA R R 673 BC 13 41 616957 S VARSHA 672 BC 14 42 619192 SRINIVASAN M 671 MBC/DNC 15 43 613436 S MADHUPRIYA 671 BC 16 45 624055 D GURUSARAN 670 BC 17 47 617133 SAKTHI K V 670 MBC/DNC 18 48 600196 D ARUN 670 BC 19 49 613404 ATITHYA G S 670 BC 20 50 619977 PUGAZHENTHI S 670 BC 21 51 616572 JAYA SURYA C 670 BC 22 53 617667 SURYANARAYANAN M S 670 BC 23 54 613771 RAANESH PRABAKAR 670 MBC/DNC 24 55 604549 R KISHORE GNANESSHWAR 670 MBC/DNC 25 56 625854 R KISHAN JOGHI 668 BC 26 57 624497 PADMAPRIYA M 668 BC 27 58 613477 UTHRA T 667 MBC/DNC 28 59 616868 AKASH K 667 BC 29 60 619463 HARIESHKUMAR N 667 BC 30 63 618602 MAHALASHMI R 666 BC Page 1 of 5 MBBS - 2020 - 2021 - SESSION LIST OF CANDIDATES ALLOTTED - ON 23.11.2020 COLLEGE : MADRAS MEDICAL COLLEGE, CHENNAI. -

History of Western Medical Education and Reservation in Tamilnadu
The International journal of analytical and experimental modal analysis ISSN NO:0886-9367 HISTORY OF WESTERN MEDICAL EDUCATION AND RESERVATION IN TAMILNADU R.PRAKASH , M.A. MPHIL., ASSISTANT PROFESSOR PG AND RESEARCH DEPARTMENT OF HISTORY SRI VASAVI COLLEGE, ERODE. TAMIL NADU, INDIA MOBILE: 9445108327 EMAIL: [email protected] ABSTRACT, Education is closely related with a nation’s development and health index is an important parameter in developing a nation. Health is not a luxury but fundamental need for a common man. This paper provides an insight into introduction and growth of Allopathic medical education in the Madras state by British and contribution of Dravidian parties to the growth of medical education in Tamil Nadu. This study also helps to know how the shifting of political scenarios and reservation policy of granting 69% to the backward communities has proved fruitful. Key Words ;Public health department-Social inequality-constitutional crisis- communal.G.O.-backward-Most Backward-Dravidar Munnetra kalagam-NEET-NEXT. The introduction of modern medicine in India was during advent of British. The first British hospital was established in 1639 A.D to treat sick solders of English East India Company. The first modern hospital in India was traced back to 1644 A.D at house of Mr. Cogan in Madras was rented for two pagodas per month (about Rs.5 today). This small rented house in 1664 private non-aided medical institutions all hospitals, dispensaries and clinics solely managed by private persons turned out to be a 105 year general hospital. After next 25 years, governer Elihu Yale gave the hospital premises in Fort St. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -

Pathology), Dip.R.C.Path, AB (AP&CP), AB(Cyto)
NAME: Dr. S. Rajendiran. DEGREE: MD (Pathology), Dip.R.C.Path, AB (AP&CP), AB(Cyto) DESIGNATION : Professor AREA OF RESEARCH EXPERTISE: Endocrine, GI, Renal, Breast and Gynaecological Pathology PROJECTS CONDUCTED (INTERNAL/ EXTERNAL) - The diagnostic value of TROP 2 , HBME 1 and CK 19 in papillary carcinoma (Dr.Arthi) - Immunohistochemical analysis of Androgen receptors in Gastric Cancer (Dr.Mounika) - Cyclin D1 expression in Ductal carcinoma of Breast (Dr.Madhumitha) - Diagnostic utility of CD56 and CK19 in papillary thyroid carcinoma from its mimickers (Dr.Gayathri) - IHC analysis of SRY related HMG bos (SOX 10) protein in breast carcinoma (Dr.Mary) - The role of SLUG as a novel marker in colorectal carcinoma (Dr.Sridevi) - SNAIL 1 as prognostic marker in colorectal carcinoma (Dr.Soundharya) - PDL-1 in colorectal carcinoma (Dr.Shivadharshini 2016) - PDL-1 expression in triple negative breast cancer (Dr.Charanya) 2016 - Lutenizing hormone releasing hormone in colorectal carcinomas (Dr.Divya S ) 2015 - Receptors for luteinizing hormone releasing hormones expressed in Human Renal Cell carcinomas (Dr.Abinizha 2015) - Folate receptor alpha expression in ovarian epithelial carcinoma (R.Manish) - Significance of Topoisomerase 2 alpha in invasive breast cancer (Dr.Arthi) 2014 - Expression of Folate receptor alpha in triple negative breast cancer & its therapeutic prognostic significance- (Dr.Mohan Krishna) 2014 AWARDS: Dr. S.J. Nagalotimath Memorial Excellence in Teaching Award – 2016, 34th Annual National CME in Pathology, June 2nd 2016, JNMC, -

COVID-19 Testing Labs
भारतीय आयु셍वज्ञि ान अनुसधं ान पररषद वा्य अनुसंधान 셍वभाग, वा्य और पररवार क쥍याण मंत्रालय, भारत सरकार Date: 20/05/2020 Total Operational (initiated independent testing) Laboratories reporting to ICMR: Government laboratories : 396 Private laboratories : 173 - Real-Time RT PCR for COVID-19 : 437 (Govt: 294 + Private: 143) - TrueNat Test for COVID-19 : 81 (Govt: 76 + Private: 05) - CBNAAT Test for COVID-19 : 51 (Govt: 26 + Private: 25) Total No. of Labs : 569 *CSIR/DBT/DST/DAE/ICAR/DRDO Laboratories. #Laboratories approved for both Real-Time RT-PCR and TrueNat/CBNAAT $Laboratories approved for both TrueNAT and CBNAAT S. Names of Test Names of Government Institutes Names of Private Institutes No. States Category 1. Andhra RT-PCR 1. Sri Venkateswara Institute of Medical Pradesh (52) Sciences, Tirupati 2. Sri Venkateswara Medical College, Tirupati 1 | P a g e भारतीय आयु셍वज्ञि ान अनुसधं ान पररषद वा्य अनुसंधान 셍वभाग, वा्य और पररवार क쥍याण मंत्रालय, भारत सरकार S. Names of Test Names of Government Institutes Names of Private Institutes No. States Category 3. Rangaraya Medical College, Kakinada 4. #Sidhartha Medical College, Vijaywada 5. Govt. Medical College, Ananthpur 6. Guntur Medical College, Guntur 7. Rajiv Gandhi Institute of Medical Sciences, Kadapa 8. Andhra Medical College, Visakhapatnam 9. Govt. Kurnool Medical College, Kurnool 10. Govt. Medical College, Srikakulam TrueNat 11. Damien TB Research Centre, Nellore 12. SVRR Govt. General Hospital, Tirupati 13. Community Health Centre, Gadi Veedhi Saluru, Vizianagaram 14. Community Health Centre, Bhimavaram, West Godavari District 15.