Chapter 2: Mate Choice & Selection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigating the Usefztlness of Evolutionary Theory for Understanding Biology and Attaimng Bioliteracy
INVESTIGATING THE USEFZTLNESS OF EVOLUTIONARY THEORY FOR UNDERSTANDING BIOLOGY AND ATTAIMNG BIOLITERACY Doreen R. Dewell B.Sc., University of Victoria, 1972 B.Ed., Universis- of British Columbia, 1992 TESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGFEE OF MASTER OF SCIENCE in the Faculty of Edwcation O Doreen R. Dewell 1998 SIMON FRASER UNINERSTTY November 1998 Al1 rights reserGd. This work may not be reproduced in whole or in part, by photocopy or other means, without permission of the author. National Library Bibliothèque natiorale du Canada Acquisitions and Acquisitions et BibIiographic Services services bibliographiques 395 Wellington Street 395. rue Wellington Ottawa ON KIA ON4 Ottawa ON KIA ON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or sell reproduire, prêter, distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/fïlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be p~tedor otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation, ABSrnCT For this qualitative study I have atîernpted to investigate the utility of evolutionary theory as a pedagogical tool to enhance understanding of biology and raise awareness of the interconnectedness of the living world. -

Brown Alumni Magazine - When Grown-Ups Fail
Brown Alumni Magazine - When Grown-Ups Fail http://www.brownalumnimagazine.com/content/view/3202/32/ July/August 2012 FEATURES THE ARTS UNDER THE ELMS SPORTS OBITUARIES MAIL ROOM FRONT PAGE BACK ISSUES When Grown-Ups Fail CLASSES By Beth Schwartzapfel '01 CONTACT US Tag it: CLASSIFIEDS ADVERTISING “T HERE ARE TWO THINGS YOU ARE NOT ALLOWED TO DO ,” HER MOTHER SAID . SUPPORT THE BAM Deborah Heiligman ’80 was sitting in her dorm WEB LINKS room, talking on the phone with her parents in Pennsylvania. She had just told them she was planning to concentrate in religious studies. “You are not allowed to be a rabbi,” her mother continued. “And you are not allowed to marry a rabbi.” So Heiligman did what any self-respecting Brown student in this situation would do: she decided to be a rabbi. Truthfully, she didn’t really want to be a rabbi. She wanted to be a writer. But she did find herself increasingly preoccupied with religion, a preoccupation that would become most apparent Headline Stories three decades later with the 2009 publication of the Brunonians Compete at biography Charles and Emma: The Darwins’ Leap the Olympics July 25, 2012 of Faith , the book that cemented Heiligman’s place as one of her generation’s best authors of books for Brown Reconsiders Paterno Honors young people. Charles and Emma is one of those 17 July 2012 books whose subject matter is so suited to its author Simmons Goes Out on a that there’s really no one else who could have Fund-Raising High written it. -

Appendix B: a Literary Heritage I
Appendix B: A Literary Heritage I. Suggested Authors, Illustrators, and Works from the Ancient World to the Late Twentieth Century All American students should acquire knowledge of a range of literary works reflecting a common literary heritage that goes back thousands of years to the ancient world. In addition, all students should become familiar with some of the outstanding works in the rich body of literature that is their particular heritage in the English- speaking world, which includes the first literature in the world created just for children, whose authors viewed childhood as a special period in life. The suggestions below constitute a core list of those authors, illustrators, or works that comprise the literary and intellectual capital drawn on by those in this country or elsewhere who write in English, whether for novels, poems, nonfiction, newspapers, or public speeches. The next section of this document contains a second list of suggested contemporary authors and illustrators—including the many excellent writers and illustrators of children’s books of recent years—and highlights authors and works from around the world. In planning a curriculum, it is important to balance depth with breadth. As teachers in schools and districts work with this curriculum Framework to develop literature units, they will often combine literary and informational works from the two lists into thematic units. Exemplary curriculum is always evolving—we urge districts to take initiative to create programs meeting the needs of their students. The lists of suggested authors, illustrators, and works are organized by grade clusters: pre-K–2, 3–4, 5–8, and 9– 12. -

Middletown Township High School North and High School South AP Language and Composition Summer Reading Assignment 2015
Middletown Township High School North and High School South AP Language and Composition Summer Reading Assignment 2015 Students are expected to read a total of six books during the summer months and complete a reader’s response journal entry for each title. Required Texts: • Eat, Shoots, Leaves by Lynne Truss • One selection from the nonfiction section Student Choice: • Four additional titles chosen from the recommendations listed below Students may read partial selections to satisfy the Student Choice four-text requirement.“Partial selections” may include articles, poems, essays, or other short works, taken from a variety of authors (see representative authors). Assignment: Students should complete a reader’s-response journal entry that includes the title and author, a few (3-5) meaningful quotations, and a short personal reflection (1-2 paragraphs) on each selection. Recommended Readings: Nonfiction In Cold Blood-Truman Capote Fast Food Nation-Eric Schlosser Woman Warrior-Maxine Hong Kingston The Glass Castle-Jeanette Wall The World is Flat-Thomas L. Friedman A Room with a View-Virginia Woolfe Black Like Me-John Howard Griffin Walden-Henry David Thoreau The Tipping Point-Malcolm Gladwell Drama Death of a Salesman- Arthur Miller Additional Representative Authors (College Board recommended) Autobiographers and Diarists Maya Angelou, James Boswell, Judith Ortiz Cofer, Charles Dana, Thomas De Quincey, Frederick Douglass, Benjamin Franklin, Lillian Hellman, Helen Keller, Maxine Hong Kingston, T. E. Lawrence, John Henry Newman, Samuel Pepys, Richard Rodriguez, Richard Wright, Malcolm X, Anzia Yezierska Biographers and History Writers Walter Jackson Bate, James Boswell, Thomas Carlyle, Winston Churchill, Wine Deloria, Jr., Leon Edel, Richard Ellmann, Shelby Foote, John Hope Franklin, Antonia Fraser, Edward Gibbon, Richard Holmes, Gerda Lerner, Thomas Macaulay, Samuel Eliot Morison, Francis Parkman, Arnold Rampersad, Simon Schama, Arthur M. -

Writerspeak Fall 2009
MFA in Writing and Literature Presents WriterSpeak Fall 2009 Duke Lecture Hall and Avram Theater • Stony Brook Southampton • Southampton NY 401 Park Ave South 2nd Fl • Stony Brook Manhattan • New York NY • Free & Open to the Public Wed Sep 17 7:00pm Duke Lecture Hall – The MFA Program: How to Get In Thesis: How to Get Out Carla Caglioti leads a lively two-part discussion: The first half for prospective students; the second half for graduate degree candidates wanting to know more about the thesis process. Caglioti is the founding Associate Director of the Stony Brook Southampton MFA in Writing and Literature program and an Assistant Dean at Stony Brook Southampton. Her English Literature PhD dissertation focuses on the rise of the field of creative writing in higher education. Wed Sep 30 7:00pm Duke Lecture Hall – Scott Snyder Scott Snyder reads fiction. Scott Snyder’s debut collection of short stories is Voodoo Heart. He has published in Zoetrope, Tin House, One-Story, Epoch, and Small Spiral Notebook. Snyder, who has taught at Co- lumbia, NYU and Sarah Lawrence, is working on a novel for the Dial Press. He is teaching the Short Story workshop this semester for the MFA program at Stony Brook Southampton. Wed Oct 14 7:00pm Duke Lecture Hall – Jules Feiffer MFA faculty member regales us with truth and humor. Jules Feiffer’s Pulitzer-winning cartoon ran for 42 years in The Village Voice. His sensibility permeates a wide range of creative work: from his Obie-winning play Little Murders, to his screenplay for Carnal Knowledge, to his Oscar-winning anti-military short subject animation Munro. -

Cite As: 22 Ecology LQ 325
FOR EDUCATIONAL USE ONLY Copr. © West 2001 No Claim to Orig. U.S. Govt. Works 22 ECGLQ 325 (Cite as: 22 Ecology L.Q. 325) Ecology Law Quarterly 1995 Review Essay *325 LAW AND THE NEW ECOLOGY: EVOLUTION, CATEGORIES, AND CONSEQUENCES Jonathan Baert Wiener [FNa] Copyright © 1995 Ecology Law Quarterly; Jonathan Baert Wiener WESTLAW LAWPRAC INDEX LIT -- Literature Reviews & Analyses Contents Introduction ................................... 325 I. Evolution in Action ............................ 329 II. Evolution in Environmental Law ................. 333 III. Changing the Metaphor for Nature ............... 338 A. Stasis ...................................... 338 B. Separatism .................................. 340 C. The Challenge of the New Ecology ............ 345 IV. Adapting Environmental Law to the New Ecology .. 350 Introduction In a fitting testament to sterling scientific journalism, the 1995 Pulitzer Prize for General Non-Fiction was awarded to a book about ecology. In The Beak of the Finch: A Story of Evolution in Our Time, [FN1] Jonathan Weiner brings to light the wealth of current empirical research demonstrating Darwinian evolution in action. Weiner focuses on the work of Rosemary and Peter Grant, two Princeton biologists who have spent much of the last twenty-odd years in the Galapagos Islands, studying the same species of finches that Charles Darwin saw over a century ago. Through Weiner's reportage, we witness the meticulous work of the Grants' jovially named "International Finch Investigation Unit" to marshal the hard evidence that Darwinian evolution is not merely a speculative theory or an ancient artifact, but that it is real and occurring now, driven ceaselessly by environmental selection pressures, in ways we humans can observe. Weiner's book is more than a biography of the Grants; it is also a tribute to Darwin, a survey of the cutting edge of ecological science today, and, most important, a bold call for a new metaphor for nature. -
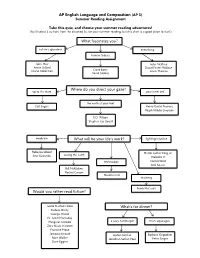
AP English Language and Composition (AP 3) What Fascinates
AP English Language and Composition (AP 3) Summer Reading Assignment Take this quiz, and choose your summer reading adventures! (You’ll select 3 authors from the attached list for your summer reading, but this chart is a good place to start.) What fascinates you? nature’s grandeur everything human foibles John Muir John McPhee Annie Dillard David Foster Wallace Diane Ackerman Dave Barry Lewis Thomas David Sedaris Where do you direct your gaze? up to the stars your inner self the earth at your feet Carl Sagan Henry David Thoreau Ralph Waldo Emerson E.O. Wilson Stephen Jay Gould medicine What will be your life’s work? fighting injustice Rebecca Skloot Martin Luther King, Jr. Atul Gawande saving the earth Malcolm X technology Cornel West bell hooks Bill McKibben Rachel Carson Nicolas Carr teaching Frank McCourt Would you rather read fiction? Leslie Marmon Silko What’s for dinner? Eudora Welty George Orwell N. Scott Momaday a juicy hamburger fresh asparagus Margaret Atwood Zora Neale Hurston Francine Prose Jamaica Kincaid Upton Sinclair Barbara Kingsolver Alice Walker Jonathan Safran Foer Peter Singer Dave Eggers Select three authors from the list below. Read at least one work by each. At least one of the works you select should be a longer, book-length work. A collection of essays counts as a longer work. The other works you select may be individual essays or speeches. The AP English Language and Composition course is primarily a nonfiction class that focuses on how and why an author says what he says. The authors on this list have been identified by the College Board as authors who skillfully employ language to achieve a specific purpose with a specific audience in mind. -

ELA Standards Guide
English Language Arts Standards Guide Acknowledgement and credit reserved to the Diocese of Owensboro, Kentucky Revised 2016 Table of Contents REVISION COMMITTEE ............................................................................................................................................. 4 NATIONAL STANDARDS AND BENCHMARKS FOR EFFECTIVE CATHOLIC SCHOOLS .............................. 5 NCTE/IRA ENGLISH LANGUAGE ARTS STANDARDS FOR 21ST CENTURY LITERACIES ........................... 6 ENGLISH/LANGUAGE ARTS PRACTICES ............................................................................................................... 7 COLLEGE AND CAREER READINESS ANCHOR STANDARDS FOR READING (R)......................................... 8 COLLEGE AND CAREER READINESS ANCHOR STANDARDS FOR WRITING (W) ...................................... 10 COLLEGE AND CAREER READINESS ANCHOR STANDARDS FOR SPEAKING AND LISTENING (SL) ... 12 COLLEGE AND CAREER READINESS ANCHOR STANDARDS FOR LANGUAGE (L)................................... 13 KINDERGARTEN ................................................................................................................................................... 15 GRADE 1 .................................................................................................................................................................. 20 GRADE 2 .................................................................................................................................................................. 26 GRADE -

Back Matter (PDF)
ABASales POBox 6599, Colorado Springs, CO80934 Phone:800/634-7736 or 719/578-0607 E-mail:[email protected] WebSite: americanbirding.org/abasales/salecatal.hfm d North Amer•a Call,6,B,6, Salesat 800/634-7736 foryour ree copy of T#e BJrder'$Calaleg specializinginbooIts, opfics, andaccessories forbirders. Ul es Birdsof Kenya and Northern Tanzania Field Guide Edition Dale A. Zimmerman, Donald A. Turner, David J. Pearson Withits modest price, small trim size and sturdy, weather- resistantbinding, this field guide is the onevolume that every travelerto Kenyaand northern Tanzania must have. The guide features124 colorplates, depicting all 1,114species in the area, and over 800 range maps. Speciallydesigned for use in the field, it isa compactversion of thewidely acclaimed Birds of Kenya and Northern Tanzania, hailed asthe mostcomprehensive, accurate, and beautiful guide ever producedfor the region. Paper$29.95 ISBN 0-691-01022-6 Cloth $39.50 ISBN 0-691-01021-8 PrincetonScience Library Edition Witha newpreface and afterword Ecoloõyand Evolution of Darwin's Finches Peter R. Grant Witha newforeword by Jonathan Weiner, ¸ Rob Couslns/BBC authorof the PufitzerPrize-winning Beak of the Finch "If yOUsomehow fell into the minority Based on the PBS TV series who didn't read the first edition, here is The Life of Birds your chanceto reform!" --Jared Diamond,author of the Pulitzer David Attenborouõh Prize-winningGuns, Germs and Steel Basedon the spectacularten-part "[This]is the bestlong-term, fine-scale TV programthat hasbeen airing studyof speciationever conducted .... on PBS, The Lifeof Birdsis The sciencePeter Grant presentshere is DavidAttenborough at his best. more interestingand importantthan Beautifullyillustrated with nearly ever." 200 colorphotographs, this book --From the forewordby JonathanWeiner will delightand informall bird 117 halftones. -
Appendix Viii | 501
APPENDIX VIII | 501 APPENDIX VIII: TEXTS ASSIGNED, 2007/2008–2017/2018 Text Assignments "Introduction" and "Welcome to Crisis Ministry" 1 from Scratch Beginnings: Me, $25, and the Search for the American Dream (2008) / Adam W. Shepard "Lisa and American Anti-Intellectualism" from 1 The Simpsons and Philosophy: The D'oh! of Homer (2001) / Aeon J. Skoble "Only Connect…" The Goals of a Liberal 2 Education (1998) / William Cronan "What Is Service Learning?" from Learning 1 through Serving: A Student Guidebook for Service-Learning Across the Disciplines (2013) / Christine M. Cress $2.00 a Day: Living on Almost Nothing in 2 America (2015) / H. Luke Shaefer and Kathryn Edin “They Say, I Say": The Moves That Matter in 1 Academic Writing (Third Edition) (2014) / Gerald Graff and Cathy Birkenstein “Why Are All the Black Kids Sitting Together in 4 the Cafeteria?” and Other Conversations About Race (1997) / Beverly Daniel Tatum 1 Dead in Attic: After Katrina (2007) / 3 Chris Rose 1000 Dollars and an Idea: Entrepreneur to 1 Billionaire (2009) / Sam Wyly 127 Hours Between a Rock and a Hard Place 1 (2004) / Aron Ralston 1491: New Revelations of the Americas Before 2 Columbus (2005) / Charles Mann 1984 (1949) / George Orwell 2 29 Gifts: How a Month of Giving Can Change 1 Your Life (2009) / Cami Walker 31 Hours (2009) / Masha Hamilton 1 365 Days, 365 Plays (2006) / Suzan-Lori Parks 1 420 Characters (2011) / Lou Beach 1 A Backpack, a Bear, and Eight Crates of Vodka 2 (2014) / Lev Golinkin 502 | TEXTS ASSIGNED, 2007/2008–2017/2018 Text Assignments A Call to Action: Women, Religion, Violence, and 1 Power (2014) / Jimmy Carter A Chance in the World: An Orphan Boy, A 3 Mysterious Past, and How He Found a Place Called Home (2012) / Steve Pemberton A Chant to Soothe Wild Elephants (2008) / 2 Jaed Coffin A Companion for Owls: Being the Commonplace 1 Book of D. -

The Pulitzer Prizes Winners An
WINNERS AND FINALISTS 1917 TO PRESENT TABLE OF CONTENTS Excerpts from the Plan of Award...................................................................................2 PULITZER PRIZES IN JOURNALISM Public Service................................................................................................................7 Reporting...................................................................................................................25 Local Reporting...........................................................................................................28 Local Reporting, Edition Time....................................................................................33 Local General or Spot News Reporting.......................................................................34 General News Reporting..............................................................................................37 Spot News Reporting...................................................................................................39 Breaking News Reporting............................................................................................40 Local Reporting, No Edition Time...............................................................................46 Local Investigative or Specialized Reporting.................................................................48 Investigative Reporting................................................................................................51 Explanatory Journalism...............................................................................................61 -

The Pulitzer Prize General Nonfiction Winners
The Pulitzer Prize General Nonfiction Winners . 2015 Elizabeth Kolbert The Sixth Extinction: An Unnatural History. An exploration of nature that forces readers to consider the threat posed by human behavior to a world of astonishing diversity. 2014 Dan Fagin Toms River: A Story of Science and Salvation. A book that deftly combines investigative reporting and historical research to probe a New Jersey seashore town’s cluster of childhood cancers linked to water and air pollution. 2013 Devil in the Grove: Thurgood Marshall, the Groveland Boys by Gilbert King. A richly detailed chronicle of racial injustice in the Florida town of Groveland in 1949, involving four black men falsely accused of rape and drawing a civil rights crusader, and eventual Supreme Court justice, into the legal battle. 2012 The Swerve: How the World Became Modern by Stephen Greenblatt. An exploration of a period of human history—the “Renaissance”—that seemed especially devoted to the pursuit of beauty and pleasure. During the Renaissance, people began to move away from supernatural explanations and began, more and more, to see the universe as consisting of matter. 2011 The Emperor of All Maladies: A Biography of Cancer by Siddhartha Mukherjee. An elegant inquiry, at once clinical and personal, into the long history of an insidious disease that, despite treatment breakthroughs, still bedevils medical science. 2010 The Dead Hand: The Untold Story of the Cold War Arms Race and Its Dangerous Legacy by David E. Hoffman. A well documented narrative that examines the terrifying doomsday competition between two superpowers and how weapons of mass destruction still imperil humankind.