Information to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of Organophosphorus Pesticide Residues in Vegetables Using Solid Phase Micro-Extraction Coupled with Gas Chromatography–flame Photometric Detector
Arabian Journal of Chemistry (2015) xxx, xxx–xxx King Saud University Arabian Journal of Chemistry www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Determination of organophosphorus pesticide residues in vegetables using solid phase micro-extraction coupled with gas chromatography–flame photometric detector Haizarul Aida Sapahin, Ahmad Makahleh *, Bahruddin Saad * School of Chemical Sciences, Universiti Sains Malaysia, 11800 Minden, Penang, Malaysia Received 17 July 2014; accepted 9 December 2014 KEYWORDS Abstract An adequate and simple analytical method based on solid-phase microextraction Organophosphorus pesticide; (SPME) followed by gas chromatography–flame photometric detection (GC–FPD) for the determi- Direct immersed-solid phase nation of eleven organophosphorus pesticide residues (i.e., ethoprophos, sulfotep, diazinon, tolclo- microextraction; fos-methyl, fenitrothion, chlorpyrifos, isofenphos, methidathion, ethion, triazophos, leptophos) in Gas chromatography–flame vegetables samples (cabbage, kale and mustard) was developed. Important parameters that influ- photometric detector; ence the extraction efficiency (i.e., fibre type, extraction modes, extraction time, salt addition, Vegetables desorption time and temperature) were systematically investigated. Four types of commercially available fibres (i.e., 50/30 lm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS), 65 lm polydimethylsiloxane/divinylbenzene (PDMS/DVB), 100 lm polydimethylsiloxane (PDMS), and 85 lm polyacrylate (PA)) were evaluated. PA fibre exhibited the best performance and was used for the rest of the studies. The optimised extraction conditions were: extraction time, 30 min at room temperature; stirring speed, 1275 rpm; salt content, 10% NaCl; desorption time and temper- ature, 11 min at 260 °C; and no pH adjustment of the sample extract. The method was validated over the range 0.1–100 lg/L. -

The List of Extremely Hazardous Substances)
APPENDIX A (THE LIST OF EXTREMELY HAZARDOUS SUBSTANCES) THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 75-86-5 ACETONE CYANOHYDRIN 500 10 1752-30-3 ACETONE THIOSEMICARBAZIDE 500/500 1,000 107-02-8 ACROLEIN 500 1 79-06-1 ACRYLAMIDE 500/500 5,000 107-13-1 ACRYLONITRILE 500 100 814-68-6 ACRYLYL CHLORIDE 100 100 111-69-3 ADIPONITRILE 500 1,000 116-06-3 ALDICARB 100/500 1 309-00-2 ALDRIN 500/500 1 107-18-6 ALLYL ALCOHOL 500 100 107-11-9 ALLYLAMINE 500 500 20859-73-8 ALUMINUM PHOSPHIDE 500 100 54-62-6 AMINOPTERIN 500/500 500 78-53-5 AMITON 500 500 3734-97-2 AMITON OXALATE 100/500 100 7664-41-7 AMMONIA 500 100 300-62-9 AMPHETAMINE 500 1,000 62-53-3 ANILINE 500 5,000 88-05-1 ANILINE,2,4,6-TRIMETHYL- 500 500 7783-70-2 ANTIMONY PENTAFLUORIDE 500 500 1397-94-0 ANTIMYCIN A 500/500 1,000 86-88-4 ANTU 500/500 100 1303-28-2 ARSENIC PENTOXIDE 100/500 1 THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 1327-53-3 ARSENOUS OXIDE 100/500 1 7784-34-1 ARSENOUS TRICHLORIDE 500 1 7784-42-1 ARSINE 100 100 2642-71-9 AZINPHOS-ETHYL 100/500 100 86-50-0 AZINPHOS-METHYL 10/500 1 98-87-3 BENZAL CHLORIDE 500 5,000 98-16-8 BENZENAMINE, 3-(TRIFLUOROMETHYL)- 500 500 100-14-1 BENZENE, 1-(CHLOROMETHYL)-4-NITRO- 500/500 500 98-05-5 BENZENEARSONIC ACID 10/500 10 3615-21-2 BENZIMIDAZOLE, 4,5-DICHLORO-2-(TRI- 500/500 500 FLUOROMETHYL)- 98-07-7 BENZOTRICHLORIDE 100 10 100-44-7 BENZYL CHLORIDE 500 100 140-29-4 BENZYL CYANIDE 500 500 15271-41-7 BICYCLO[2.2.1]HEPTANE-2-CARBONITRILE,5- -

Malathion Human Health and Ecological Risk Assessment Final Report
SERA TR-052-02-02c Malathion Human Health and Ecological Risk Assessment Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0012 SERA Internal Task No. 52-02 Submitted by: Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com May 12, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables ................................................................................................................................. vi List of Appendices ......................................................................................................................... vi List of Attachments........................................................................................................................ vi ACRONYMS, ABBREVIATIONS, AND SYMBOLS ............................................................... vii COMMON UNIT CONVERSIONS AND ABBREVIATIONS.................................................... x CONVERSION OF SCIENTIFIC NOTATION .......................................................................... -

744 Hydrolysis of Chiral Organophosphorus Compounds By
[Frontiers in Bioscience, Landmark, 26, 744-770, Jan 1, 2021] Hydrolysis of chiral organophosphorus compounds by phosphotriesterases and mammalian paraoxonase-1 Antonio Monroy-Noyola1, Damianys Almenares-Lopez2, Eugenio Vilanova Gisbert3 1Laboratorio de Neuroproteccion, Facultad de Farmacia, Universidad Autonoma del Estado de Morelos, Morelos, Mexico, 2Division de Ciencias Basicas e Ingenierias, Universidad Popular de la Chontalpa, H. Cardenas, Tabasco, Mexico, 3Instituto de Bioingenieria, Universidad Miguel Hernandez, Elche, Alicante, Spain TABLE OF CONTENTS 1. Abstract 2. Introduction 2.1. Organophosphorus compounds (OPs) and their toxicity 2.2. Metabolism and treatment of OP intoxication 2.3. Chiral OPs 3. Stereoselective hydrolysis 3.1. Stereoselective hydrolysis determines the toxicity of chiral compounds 3.2. Hydrolysis of nerve agents by PTEs 3.2.1. Hydrolysis of V-type agents 3.3. PON1, a protein restricted in its ability to hydrolyze chiral OPs 3.4. Toxicity and stereoselective hydrolysis of OPs in animal tissues 3.4.1. The calcium-dependent stereoselective activity of OPs associated with PON1 3.4.2. Stereoselective hydrolysis commercial OPs pesticides by alloforms of PON1 Q192R 3.4.3. PON1, an enzyme that stereoselectively hydrolyzes OP nerve agents 3.4.4. PON1 recombinants and stereoselective hydrolysis of OP nerve agents 3.5. The activity of PTEs in birds 4. Conclusions 5. Acknowledgments 6. References 1. ABSTRACT Some organophosphorus compounds interaction of the racemic OPs with these B- (OPs), which are used in the manufacturing of esterases (AChE and NTE) and such interactions insecticides and nerve agents, are racemic mixtures have been studied in vivo, ex vivo and in vitro, using with at least one chiral center with a phosphorus stereoselective hydrolysis by A-esterases or atom. -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

The Benefit of Combinations of Oximes for the Ability of Antidotal Treatment
Caisberger et al. BMC Pharmacology and Toxicology (2018) 19:35 https://doi.org/10.1186/s40360-018-0227-0 RESEARCH ARTICLE Open Access The benefit of combinations of oximes for the ability of antidotal treatment to counteract sarin-induced brain damage in rats Filip Caisberger1, Jaroslav Pejchal2, Jan Misik2, Jiri Kassa2, Martin Valis1 and Kamil Kuca3,4* Abstract Background: The aim of our study was to compare the ability of two combinations of oximes (HI-6 + trimedoxime and HI-6 + K203) with atropine to counteract acute sarin-induced brain damage with the efficacy of antidotal treatment involving single oxime (HI-6) and atropin using in vivo methods. Methods: Brain damage and neuroprotective effects of antidotal treatment were evaluated in rats poisoned with sarin at a sublethal dose (108 μg/kg i.m.; 90% LD50) using histopathological, Fluoro-Jade B and Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis 24 h after sarin administration. Results: Both combinations of oximes reduce the number of rats that died before the end of experiment compared to non-treated sarin poisoning and sarin poisoning treated with HI-6 and atropine. In the case of treatment of sarin poisoning with HI-6 in combination with K203, all rats survived till the end of experiment. HI-6 with atropine was able to reduce sarin-induced brain damage, however, both combinations were slightly more effective. Conclusions: The oxime HI-6 in combination with K203 and atropine seems to be the most effective. Thus, both tested oxime combinations bring a small benefit in elimination of acute sarin-induced brain damage compared to single oxime antidotal therapy. -

Carbaryl Human Health and Ecological Risk Assessment Revised Final Report
SERA TR-052-01-05a Carbaryl Human Health and Ecological Risk Assessment Revised Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0009 SERA Internal Task No. 52-01 Submitted by: Patrick R. Durkin and Cynthia King Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com February 9, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables .................................................................................................................................. v List of Attachments........................................................................................................................ vi List of Appendices ......................................................................................................................... vi COMMON UNIT CONVERSIONS AND ABBREVIATIONS................................................... ix CONVERSION OF SCIENTIFIC NOTATION ............................................................................ x EXECUTIVE SUMMARY .......................................................................................................... -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Hazard Assessment QUANTITATIVE STRUCTURE-ACTIVITY RELATIONSHIPS for PREDICTING the TOXICITY of PESTICIDES in AQUATIC SYSTEMS with SEDIMENT
Environmental Toxicolog~and Chemistry, Vol. 12, pp. 1307-1318, 1993 0730-7268/93 $6.00 + .OO Printed in the USA. Pergamon Press Ltd. Copyright O 1993 SETAC Hazard Assessment QUANTITATIVE STRUCTURE-ACTIVITY RELATIONSHIPS FOR PREDICTING THE TOXICITY OF PESTICIDES IN AQUATIC SYSTEMS WITH SEDIMENT S.W. FISHER,*?M. J. LYDY,$ J. BARGER~and P.E LANDRUMIJ ?Department of Entomology and $Department of Zoology, Ohio State University, Columbus, Ohio 43210 8U.S Department of Agriculture Forest Service, 359 Main Road, Delaware, Ohio 43015 IlGreat Lakes Environmental Research Laboratory, 2205 Commonwealth Boulevard, Ann Arbor, Michigan 48105-1593 (Received 1 November 1991; Accepted 13 October 1992) Abstract -The toxicity of a series of organophosphorus (OP) and carbamate insecticides was mea- sured against the midge Chironomus riparius in aquatic systems with and without sediment. Five molecular descriptors (molecular volume, Henry's law constant, n-octanol/water partition coeffi- cient (KO,), molecular connectivity, and linear solvation energy) were used in regression analysis as potential predictors of insecticidal activity. The regressions were conducted for each descriptor against toxicity values for the series of chemicals. Molecular volume and Henry's law constant showed no relationship with toxicity. However, log KO, was moderately successful in describing the effect of sediment on toxicity (r2 = 0.508). Predic- tion of toxicity was substantially improved when a linear solvation energy (LSE) or molecular con- nectivity (MC) model was used in regressions. In multiple regressions conducted on carbamates and OPs separately, use of MC or LSE parameters explained up to 95.8% of the variability in toxicity. Based on the results of regression analyses, sorptive interactions between these insecticides and sed- iment apparently dominate the processes affecting the toxicity of these compounds when sediment is present. -

View Is Primarily on Addressing the Issues of Non-Permanently Charged Reactivators and the Development of Treatments for Aged Ache
Design, Synthesis, and Evaluation of Therapeutics for the Treatment of Organophosphorus Poisoning by Nerve Agents and Pesticides Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Andrew Joseph Franjesevic Graduate Program in Chemistry The Ohio State University 2019 Dissertation Committee Professor Christopher M. Hadad, Advisor Professor Thomas J. Magliery Professor David Nagib Professor Jonathan R. Parquette Copyrighted by Andrew Joseph Franjesevic 2019 2 Abstract Organophosphorus (OP) compounds, both pesticides and nerve agents, are some of the most lethal compounds known to man. Although highly regulated for both military and agricultural use in Western societies, these compounds have been implicated in hundreds of thousands of deaths annually, whether by accidental or intentional exposure through agricultural or terrorist uses. OP compounds inhibit the function of the enzyme acetylcholinesterase (AChE), and AChE is responsible for the hydrolysis of the neurotransmitter acetylcholine (ACh), and it is extremely well evolved for the task. Inhibition of AChE rapidly leads to accumulation of ACh in the synaptic junctions, resulting in a cholinergic crisis which, without intervention, leads to death. Approximately 70-80 years of research in the development, treatment, and understanding of OP compounds has resulted in only a handful of effective (and approved) therapeutics for the treatment of OP exposure. The search for more effective therapeutics is limited by at least three major problems: (1) there are no broad scope reactivators of OP-inhibited AChE; (2) current therapeutics are permanently positively charged and cannot cross the blood-brain barrier efficiently; and (3) current therapeutics are ineffective at treating the aged, or dealkylated, form of AChE that forms following inhibition of of AChE by various OPs. -
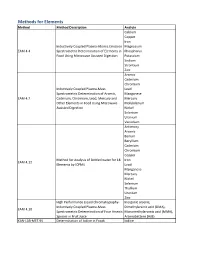
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and