The Conus Medullaris: a Comprehensive Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intramedullary Cystic Lesions Ofthe Conus Medullaris
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.31.2.106 on 1 April 1968. Downloaded from J. Neurol. Neurosurg. Psychiat., 1968, 31, 106-109 Intramedullary cystic lesions of the conus medullaris SAMI I. NASSAR, JAMES W. CORRELL, AND EDGAR M. HOUSEPIAN From the Department of Neurosurgery, College ofPhysicians and Surgeons, Columbia University, and the Neurological Institute of the Columbia-Presbyterian Medical Center, New York, U.S.A. Intramedullary cystic lesions of the conus medullaris of the aetiology, these cysts may simulate the clinical are rare. Although an extensive literature describes picture of syringomyelia. syringomyelia as being a frequent basis for cystic The cases of cysts of the conus medullaris re- cervico-thoracic lesions it is apparent that this ported here simulated the clinical picture of does not occur frequently in the lumbosacral region syringomyelia, tumour, or lumbar disc disease. (Kirgis and Echols, 1949; Netsky, 1953; Rand and The radiographic findings in each case were inter- Rand, 1960; Love and Olafson, 1966). Poser (1956), preted as indicating the presence ofan intramedullary in a review of 234 cases of syringomyelia, found tumour. The correct diagnosis was made in each that the cavity extended into the lumbosacral region case only at operation. in only 12-6% and in only five cases were the Protected by copyright. cavities restricted to the lumbosacral segments. Some authors (Thevenard, 1942; Andre, 1951) CASE REPORTS question the occurrence of syringomyelia in the lower spinal cord. Nevertheless a high incidence CASE 1 (F.T., NO. 179 16 92) A 22-year-old negro male of constitutional defects has been noted among was admitted complaining of weakness and pain in the syringomyelia patients and members of their legs for three years. -

Split Spinal Cord Malformations in Children
Split spinal cord malformations in children Yusuf Ersahin, M.D., Saffet Mutluer, M.D., Sevgül Kocaman, R.N., and Eren Demirtas, M.D. Division of Pediatric Neurosurgery, Department of Neurosurgery, and Department of Pathology, Ege University Faculty of Medicine, Izmir, Turkey The authors reviewed and analyzed information on 74 patients with split spinal cord malformations (SSCMs) treated between January 1, 1980 and December 31, 1996 at their institution with the aim of defining and classifying the malformations according to the method of Pang, et al. Computerized tomography myelography was superior to other radiological tools in defining the type of SSCM. There were 46 girls (62%) and 28 boys (38%) ranging in age from less than 1 day to 12 years (mean 33.08 months). The mean age (43.2 months) of the patients who exhibited neurological deficits and orthopedic deformities was significantly older than those (8.2 months) without deficits (p = 0.003). Fifty-two patients had a single Type I and 18 patients a single Type II SSCM; four patients had composite SSCMs. Sixty-two patients had at least one associated spinal lesion that could lead to spinal cord tethering. After surgery, the majority of the patients remained stable and clinical improvement was observed in 18 patients. The classification of SSCMs proposed by Pang, et al., will eliminate the current chaos in terminology. In all SSCMs, either a rigid or a fibrous septum was found to transfix the spinal cord. There was at least one unrelated lesion that caused tethering of the spinal cord in 85% of the patients. -

The Spinal Cord Is a Nerve Column That Passes Downward from Brain Into the Vertebral Canal
The spinal cord is a nerve column that passes downward from brain into the vertebral canal. Recall that it is part of the CNS. Spinal nerves extend to/from the spinal cord and are part of the PNS. Length = about 17 inches Start = foramen magnum End = tapers to point (conus medullaris) st nd and terminates 1 –2 lumbar (L1-L2) vertebra Contains 31 segments à gives rise to 31 pairs of spinal nerves Note cervical and lumbar enlargements. cauda equina (“horse’s tail”) –collection of spinal nerves at inferior end of vertebral column (nerves coming off end of spinal cord) Meninges- cushion and protected by same 3 layers as brain. Extend past end of cord into vertebral canal à spinal tap because no cord A cross-section of the spinal cord resembles a butterfly with its wings outspread (gray matter) surrounded by white matter. GRAY MATTER or “butterfly” = bundles of cell bodies Posterior (dorsal) horns=association or interneurons (incoming somatosensory information) Lateral horns=autonomic neurons Anterior (ventral) horns=cell bodies of motor neurons Central canal-found within gray matter and filled with CSF White Matter: 3 Regions: Posterior (dorsal) white column or funiculi – contains only ASCENDING tracts à sensory only Lateral white column or funiculi – both ascending and descending tracts à sensory and motor Anterior (ventral) white column or funiculi – both ascending and descending tracts à sensory and motor All nerve tracts made of mylinated axons with same destination and function Associated Structures: Dorsal Roots = made -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

227 INTRODUCTION: Diastematomyelia (Also Known As a Split Cord Malformation) Is a Rare Dysraphic Lesion of the Spinal Cord in W
Role of rehabilitation a case of diastematomyelia Stanescu Ioana¹, Kallo Rita¹, Bulboaca Adriana³, Dogaru Gabriela ² 1.Rehabilitation Hospital Cluj, Neurology Department 2.Rehabilitation Hospital Cluj, Physical Medicine and Rehabilitation Department 3.University of Medecine and Pharmacy Cluj - Physiopathology Department Balneo Research Journal DOI: http://dx.doi.org/10.12680/balneo.2017.156 Vol.8, No.4, December 2017 p: 227 – 230 Corresponding author: Gabriela Dogaru, E-mail address: [email protected] Abstract Diastematomyelia (split cord malformation) is a rare dysraphic lesion in which a part of the spinal cord is split in the sagittal plane into two hemicords, a bony, cartilagenous or fibrous spur projecting through the dura mater is visible in 33% of cases. Vertebral anomalies (spina bifida) are common. It occurs usually between D9 and S1. Classificatin includes two types: type 1 with a duplicated dural sac, with common midline spur, usually symptomatic, and type 2 with a single dural sac and usually less symptomatic. The majority of patients are presenting with tethered cord syndrome (neurologic deficits in the lower limbs and perineum). MRI is the modality of choice for diagnosis. In symptomatic cases, surgical release of the cord and resection of spur with repair of dura are performed, with good results. We present a case of a pauci-symptomatic type 1 dyastematomyelia, manifested by intermittent and resistant lumbar pain, in which physiotherapy during rehabilitation program have shown to improve pain intensity. Key words: spinal cord malformation, dyastematomyelia, lumbar spine, INTRODUCTION: Diastematomyelia (also Intramedullary tumours associated with known as a split cord malformation) is a rare diastematomyelia have been rarely described and dysraphic lesion of the spinal cord in which a part associated conditions like a tethered cord, inclusion of the spinal cord is split in the sagittal plane into dermoid, lipoma, syringo-hydromyelia and Chiari two hemicords. -
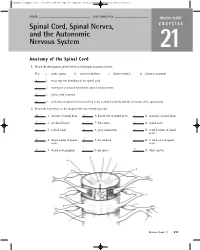
Spinal Cord, Spinal Nerves, and the Autonomic Nervous System
ighapmLre21pg211_216 5/12/04 2:24 PM Page 211 impos03 302:bjighapmL:ighapmLrevshts:layouts: NAME ___________________________________ LAB TIME/DATE _______________________ REVIEW SHEET Spinal Cord, Spinal Nerves, exercise and the Autonomic Nervous System 21 Anatomy of the Spinal Cord 1. Match the descriptions given below to the proper anatomical term: Key: a. cauda equina b. conus medullaris c. filum terminale d. foramen magnum d 1. most superior boundary of the spinal cord c 2. meningeal extension beyond the spinal cord terminus b 3. spinal cord terminus a 4. collection of spinal nerves traveling in the vertebral canal below the terminus of the spinal cord 2. Match the key letters on the diagram with the following terms. m 1. anterior (ventral) hornn 6. dorsal root of spinal nervec 11. posterior (dorsal) horn k 2. arachnoid materj 7. dura materf 12. spinal nerve a 3. central canalo 8. gray commissure i 13. ventral ramus of spinal nerve h 4. dorsal ramus of spinald 9. lateral horne 14. ventral root of spinal nerve nerve g l 5. dorsal root ganglion 10. pia materb 15. white matter o a b n c m d e l f g k h j i Review Sheet 21 211 ighapmLre21pg211_216 5/12/04 2:24 PM Page 212 impos03 302:bjighapmL:ighapmLrevshts:layouts: 3. Choose the proper answer from the following key to respond to the descriptions relating to spinal cord anatomy. Key: a. afferent b. efferent c. both afferent and efferent d. association d 1. neuron type found in posterior hornb 4. fiber type in ventral root b 2. -
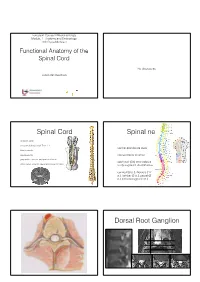
Functional Anatomy of the Spinal Cord
European Course in Neuroradiology Module 1 - Anatomy and Embryology 16th Cycle Module 1 Functional Anatomy of the Spinal Cord No disclosures Johan Van Goethem Spinal Cord Spinal nerves • vertebral canal • conus medullaris: adult Th12- L1 • ventral and dorsal roots • filum terminale • cauda equina • intervertebral foramen • gray matter: anterior and posterior horns • each pair (31) innervates a • white matter: anterior, lateral and posterior tracts body segment: dermatomes • cervical (8 p.), thoracic (12 p.), lumbar (5 p.), sacral (5 p.) and coccygeal (1 p.) Dorsal Root Ganglion Dorsal Root Ganglion Elliot S. Krames et al 2014 Tiantian Guo et al 2019 Spinal cord anatomy Spinal cord anatomy Source: Prometheus • 3 ascending pathways Anatomical atlas • 2 descending pathways Source: Wikipedia 40-year-old woman So what is this? • gradual and uniform onset of • Lichtheim's disease • diminished pressure, vibration and touch sense • Lou Gehrig's disease • tingling and numbness of legs, arms and trunk that progressively worsens • Lyme disease • pain and temperature sense are normal • Devic’s disease • motor function is preserved, but slight ataxia And the answer is ... Let’s go back ... • gradual and uniform onset of • Lichtheim's disease • diminished pressure, vibration and touch sense • Lou Gehrig's disease • tingling and numbness of legs, arms and trunk that progressively worsens • Lyme disease • pain and temperature sense are normal • Devic’s disease • motor function is preserved, but slight ataxia Spinothalamic tract Spinothalamic tract • 3 -

Definitions of Traumatic Conus Medullaris and Cauda
Spinal Cord (2017) 55, 886–890 & 2017 International Spinal Cord Society All rights reserved 1362-4393/17 www.nature.com/sc ORIGINAL ARTICLE Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review E Brouwers1, H van de Meent2, A Curt3, B Starremans4, A Hosman5 and R Bartels1 Study design: A systematic review. Objectives: Conus medullaris syndrome (CMS) and cauda equina syndrome (CES) are well-known neurological entities. It is assumed that these syndromes are different regarding neurological and functional prognosis. However, literature concerning spinal trauma is ambiguous about the exact definition of the syndromes. Methods: A MEDLINE, EMBASE and Cochrane literature search was performed. We included original articles in which clinical descriptions of CMS and/or CES were mentioned in patients following trauma to the thoracolumbar spine. Results: Out of the 1046 articles, we identified 14 original articles concerning patients with a traumatic CMS and/or CES. Based on this review and anatomical data from cadaveric and radiological studies, CMS and CES could be more precisely defined. Conclusion: CMS may result from injury of vertebrae Th12–L2, and it involves damage to neural structures from spinal cord segment Th12 to nerve root S5. CES may result from an injury of vertebrae L3–L5, and it involves damage to nerve roots L3–S5. This differentiation between CMS and CES is necessary to examine the hypothesis that CES patients tend to have a better functional outcome. Spinal Cord (2017) 55, 886–890; doi:10.1038/sc.2017.54; published online 23 May 2017 INTRODUCTION described clinical symptoms that were caused by compression of the Traumatic injuries of the thoracolumbar spine can result in conus CE and named this as ‘cauda equina compression syndrome’.20 From medullaris syndrome (CMS) or cauda equina syndrome (CES). -

Clinical Article Results of the Section of the Filum Terminale in 20 Patients with Syringomyelia, Scoliosis and Chiari Malformat
Acta Neurochir (Wien) (2005) 147: 515–523 DOI 10.1007/s00701-005-0482-y Clinical Article Results of the section of the filum terminale in 20 patients with syringomyelia, scoliosis and Chiari malformation M. B. Royo-Salvador1, J. Sole´-Llenas2, J. M. Dome´nech3, and R. Gonza´lez-Adrio4 1 Barcelona Neurological Institute, Barcelona, Spain 2 Department of Neuroradiology, Universitat Autoonoma de Barcelona, Barcelona, Spain 3 Department of Anatomy, Universitat Autoonoma de Barcelona, Barcelona, Spain 4 FIATC Foundation, Barcelona, Spain Published online February 24, 2005 # Springer-Verlag 2005 Summary ing of a tight and thick filum. Jones and Love [14] used Background. Spinal cord traction caused by a tight filum terminale the term ‘tight filum terminale syndrome’ and reported may be considered a pathogenic mechanism involved in the develop- six patients with spina bifida occulta the symptoms of ment of syringomyelia, the Chiari malformation (type I) and scoliosis. which were attributed to an anchored conus medullaris. Section of the filum terminale is proposed as a useful surgical approach In all cases, symptomatic improvement was obtained in these conditions. Methods. Between April 1993 and July 2003, a total of 20 patients after intradural lumbosacral exploration and resection (8 men and 12 women) with a mean age of 33.5 years underwent section of the filum terminale. Hamilton [10] and Roth [25, of the filum terminale with or without opening of the dural sac through 26] established the hypothesis that stretching of the a standard sacrectomy. Eight patients suffered from scoliosis, 5 from syringomyelia, 2 from Chiari malformation and 5 with a combination of spinal cord was involved in the aetiopathogenesis these conditions. -

The Spinal Cord and Spinal Nerves
14 The Nervous System: The Spinal Cord and Spinal Nerves PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The Central Nervous System (CNS) consists of: • The spinal cord • Integrates and processes information • Can function with the brain • Can function independently of the brain • The brain • Integrates and processes information • Can function with the spinal cord • Can function independently of the spinal cord © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • 45 cm in length • Passes through the foramen magnum • Extends from the brain to L1 • Consists of: • Cervical region • Thoracic region • Lumbar region • Sacral region • Coccygeal region © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • Consists of (continued): • Cervical enlargement • Lumbosacral enlargement • Conus medullaris • Cauda equina • Filum terminale: becomes a component of the coccygeal ligament • Posterior and anterior median sulci © 2012 Pearson Education, Inc. Figure 14.1a Gross Anatomy of the Spinal Cord C1 C2 Cervical spinal C3 nerves C4 C5 C 6 Cervical C 7 enlargement C8 T1 T2 T3 T4 T5 T6 T7 Thoracic T8 spinal Posterior nerves T9 median sulcus T10 Lumbosacral T11 enlargement T12 L Conus 1 medullaris L2 Lumbar L3 Inferior spinal tip of nerves spinal cord L4 Cauda equina L5 S1 Sacral spinal S nerves 2 S3 S4 S5 Coccygeal Filum terminale nerve (Co1) (in coccygeal ligament) Superficial anatomy and orientation of the adult spinal cord. The numbers to the left identify the spinal nerves and indicate where the nerve roots leave the vertebral canal. -

Brain, Spinal Cord and Spinal Tracts Definitions to Bring Home
Brain, spinal cord and spinal tracts Definitions to bring home Nerve Ganglion Neuron Nucleus Tract Collection neurons Collection of nerve A single cell Collection of Collection of axons that transmits cell bodies in the transmitting nerve cell bodies in traveling up or sensation or motor PNS, typically linked electrical impluses. the CNS, typically down the spinal impulses depending by synapses. linked by synapses. cord, depending on the function and Location: both on function destination. Location: PNS Location: CNS and destination. Location: PNS Location: CNS ANS Characteristic Sympathetic Parasympathetic Nuclei of CNIII, VII, IX, X Origin of Preganglionic Nerve Nuclei of Spinal Cord segs T1-T12, L1-L3 Spinal Cord S2-S4 Length of Preganglionic n. Axon Short Long Receptor type in ganglion Nicotinic Nicotinic Neurotransmitter in ganglion Acetylcholine Acetylcholine Length of Postganglionic nerve axon Long Short Effector Organs Smooth + cardiac muscle, glands Smooth + cardiac muscle, glands Neurotransmitter in effector organs Norepinephrine (ACh in glands) Acetylcholine Receptor types in effector organs A1, A2, B1, B2, B3 M1, M2, M3 BRAIN 1. CEREBRUM • Higher brain functions 2. CEREBELLUM • Little brain • Posture and balance • Coordinate movement 3. BRAIN STEM • Connects cerebrum/cerebellum and spinal cord • CN III – XII nuclei • 3 parts: • Midbrain / Mesencephalon • Pons • Medulla oblongata CEREBRUM • Left and right hemisphere • 4 lobes: • Frontal = motor + speech • Parietal = sensory (homunculus) • Temporal = memory + understanding • Occipital = vision SPINAL CORD • Signal to and from the brain • Grey matter = cell bodies + unmyelinated axons • White matter = myelinated axons • Central canal = CSF • Bell-Magendie Law • Ventral = motor • Ventral horn • Dorsal = sensory • Dorsal root ganglia Spinal cord terminations • Spinal cord: lower border of L1-L3 • Subarachnoid space (with CSF): lower border of S2 • Cauda equina: L3 (adult age) • Filum terminale: fibrous string 3 main functions of the spinal cord: 1. -

A&P Spinal PNS A
Chapter 13 Spinal Cord & PNS THE SPINAL CORD The SPINAL CORD extends from the forman magnum down to about L1, L2. At the distal end is the CONUS MEDULLARIS. This is a blunted conical terminus at L1. The spincal cord has MENINGES that are the same as around the brain, except there is no periosteal layer of the dura mater. The reason for this, is because the vertebral column has to bend and felx. The spinal cord is not fixed to the vertebral canal. Down from the conos medullaris is the FILUM TERMINALE. It is a continuation of pia mater beyond the conus medullaris and it anchors the spinal cord to the coccyx. DENTICULATE LIGAMENTS anchor the spinal cord laterally. These are lateral projections of pia mater at each spinal level. SPINAL ENLARGEMENTS are areas where the spine gets thicker. There are more neurons in these regions because they serve limbs. There are two of them: 1. Cervical 2. Lumbar CAUDA EQUINA is a broom-like collection of nerve roots extending inferior to the conus medullaris. It forms because the vertebral column grows faster and longer than the spinal cord. So, the nerves are pulled inferiorly with intervertebral foramina. This area is where spinal taps are done! X-SECTIONAL ANATOMY OF THE SPINAL CORD The ANTERIOR MEDIAN FISSURE is a deep longitudinal groove along the anterior surface The POSTERIOR MEDIAL SULCUS is a shallower groove along the posterior surface GRAY MATTER is the internal butterfly-shaped region. The wings have horns! The ANTERIOR GRAY HORNS contain somatic motor neurons (go to skeletal muscle).